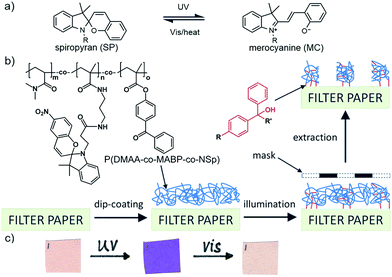
Preparation of photochromic paper, using fibre-attached spiropyran polymer networks†
W. Li,
S. Trosien,
H. Schenderlein,
M. Graf and
M. Biesalski*
Laboratory of Macromolecular Chemistry and Paper Chemistry, Department of Chemistry, Technische Universitaet Darmstadt, 64287 Darmstadt, Germany. E-mail: biesalski@tu-darmstadt.de
First published on 9th November 2016
Abstract
Photochromic paper was prepared by covalent immobilisation of functional polymer networks with spiropyran moieties on paper sheets. The chromatic response of spiropyran molecules in the system can be dynamically controlled by simple damping techniques using multilayered paper samples. Based on the colour change of the photochromic paper upon different UV doses, a first proof-of-concept application for a paper-based colourimetric UV sensor was developed.
Stimuli-responsive polymeric materials are of great interest for scientific research, and possible applications include the development of sensors, actuators and other (multi)functional materials.1–6 Among various stimuli that can trigger a desired change in the properties of the material, light is of particular interest because the responding system can be remotely and accurately controlled in a fast, non-invasive, and non-diffusively restricted way under mild conditions (without sophisticated catalysts or special solvents, and at ambient temperature).7–9 Among various light-responsive molecules, spiropyran has become a research hotspot owing to its unique properties. It consists of an indoline and a chromene moiety, which are linked by a spiro carbon atom in an orthogonal fashion. When spiropyran (SP) is illuminated with UV light, a photo-cleavage of the 6-membered pyran moiety occurs and a merocyanine (MC) isomer is generated. If the MC molecule is treated with visible light or heat, it transforms back to the SP form (Fig. 1a).10–17 The two isomers exhibit significantly different physicochemical properties.18,19 The most obvious difference is their chromatic appearance. The SP form is colourless, while the MC form is purple. Therefore, the photochromic properties of spiropyran-based materials can be conveniently determined by colour changes. To realize the “naked-eye readout” of colour changes for further applications, the choice of substrate is very important. Paper as a substrate is highly interesting owing to the following properties:20–27 (1) its rough and porous structure results in a large interfacial area, which can increase the number of immobilised functional molecules and lead to a high sensitivity; (2) it is light-weight, which is highly desirable for portable devices; (3) it is an opaque material and provides a colourless, bright and high-contrast background so that it can be easily used for test strips with “naked-eye readout”; and (4) it is chemically stable and the surface of the fibres of the paper sheet can be modified with organic functional groups by various methods, which makes it a widely used flexible substrate in our daily lives.
To date, there have been few publications on spiropyran-functionalised paper substrates. In Tian's group, they modified cellulose pulp using spiropyran derivatives followed by a conventional papermaking procedure.28 Such a spiropyran-grafted cellulose paper shows enhanced fluorescent properties. However, the authors reported that reversible switching is not possible. In Mahdavian's group, they first synthesized spiropyran-functionalised latex particles by emulsion polymerization.29–31 The obtained latex was then incorporated into cellulose paper, either by coating of the latex onto commercial filter paper, or by blending the particles with cellulose pulp and casting the suspension in a Petri disk, with subsequent drying to yield the paper substrates. The modified paper shows photochromic and solvatochromic behaviour upon UV illumination. However, the authors also mentioned limitations with respect to the integrity of the structure of the paper sheets if post-modification of the material was performed.
In the present work, we follow a different, alternative strategy: photochromic paper was prepared by generation and chemical immobilisation of spiropyran-containing polymer networks on paper by dip-coating and consecutive photocrosslinking of the polymers. After immobilisation of the polymers onto paper substrates, the photochromic behaviour of spiropyran molecules in the system was investigated. Despite a simple colourless-purple change in the colour of the functional paper strips, in these studies, we addressed the design of paper-strips where a controlled deceleration in the colour change of the modified paper allows a high sensitivity and a dynamic range of measurements. Since any chromatic response depends on the number of photons that reach the surface, we therefore developed a simple “damping strategy”, which effectively decreases the speed of the photo-induced switching process. Finally, based on the developed standard colour change of the spiropyran-functionalised paper upon the application of different UV doses, a first proof-of-concept application as a UV sensor was created.
For the preparation of our paper-based photochromic material, we first synthesized functional terpolymers with photo-cross-linkable benzophenone groups (1.9 ± 0.1 mol%) as well as light-responsive spiropyran moieties (4.5 ± 0.2 mol%) that were tethered to a non-toxic poly(N,N′-dimethylacrylamide) (PDMAA) matrix by conventional free radical polymerization.32 The polymers were dissolved in an organic solvent (THF), and transferred onto model paper sheets (Roth 15A filter paper) by a dip-coating process. Subsequently, the fibre-adsorbed macromolecules were crosslinked using UV light (λ = 254 nm, with an energy of 400 mJ cm−2). This procedure allows for the cross-linking of the polymeric material in the adsorbed film, and simultaneously, it covalently attaches the polymers to the organic paper fibres.32–37 When a photolithography mask is used during UV illumination, the polymers can be attached at defined locations, i.e., illuminated areas. Polymers that are located in shaded areas do not undergo photo-reaction and can be easily washed out via Soxhlet extraction (Fig. 1b).33,34
After preparation, the surface morphology of the spiropyran-modified paper was investigated by scanning electron microscopy (SEM) (Fig. S4†). The similar morphology compared with that of the unmodified paper indicates that the integrity of the paper substrate is retained during dip-coating, drying and UV cross-linking of the sheets. After modification, the chemical composition the functional paper was determined by UATR-FTIR spectroscopy (Fig. S5†). Since any chromatic response to UV light strongly relies on the concentration of paper-attached spiropyran molecules, it is important to control and quantify the amount of immobilised polymers. Because paper-adsorbed polymers can be bound in an almost quantitative manner by UV light-induced cross-linking,33 a simple way to control the amount of paper-attached macromolecules is by varying the concentration of the polymers in the dip-coating step. As shown in the ESI (Fig. S6†), by changing the polymer concentration in solution from 0 to approximately 30 mg mL−1, the immobilised amount of functional polymers increases from 0 to approximately 28 mg cross-linked polymers per gram of fibres (the dry weight). The amount of dry paper-attached polymers was determined by elemental analysis, since the polymers carry an abundant number of nitrogen-atoms while the paper fibres do not, and since other techniques, such as spectroscopy or gravimetric analysis are not easy to perform here. Note that at dip-coating concentrations that exceed 30 mg mL−1, it becomes difficult to obtain a homogeneous coating owing to a high viscosity of the polymer solution. To ensure a homogeneous paper coating, a high amount of fibre-immobilised functional polymers and a maximum degree of sensitivity, a polymer concentration of 30 mg mL−1 was chosen for the preparation of all further samples. The prepared samples were stored in the dark under norm-climate conditions prior to further investigation.
After preparation, we studied the chromatic response of the functional paper upon UV illumination. The UV-generated MC molecule has a large π-electron system, which leads to a strong absorption in the visible light region. Consequently, MC is strongly coloured (purple) while SP is almost colourless.38–42 As observed in Fig. 1c, the spiropyran-functionalised paper is almost colourless (light rose); it turns purple after exposure to UV light and can be switched back by visible light illumination.
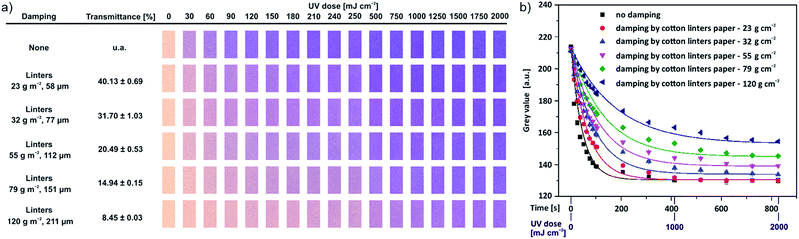
Additionally, the UV-induced colour change can be described as a function of the number of generated MC groups within the paper-attached polymers, which depends on the applied UV dose.43,44 To study this process in our material, separate spiropyran-functionalised paper strips (1 × 1 cm2) were prepared and used to obtain an exact colour change for each UV dose (Biolink UV chamber, λ = 365 nm, 0–2000 mJ cm−2). After UV illumination, a digital image of the sample was taken using an office scanner. Note that, the digitalized image obtained from an office scanner is comparable to that from a scientific camera (Fig. S9†). Due to the simple-to-operate property, we adopted the office scanner for all further experiments. Meanwhile, the unmodified paper and the reference polymer (P(DMAA-co-MABP))-modified paper were analysed in a similar fashion to demonstrate that the colour change is exclusively derived from the spiropyran moieties. Fig. 2a (the first horizontal row of images) shows that the colour intensity of the UV-illuminated spiropyran-functionalised paper increases as the UV dose increases until a stable value is reached. Note that the colour change of the spiropyran-functionalised paper can be directly observed by the naked eye after UV illumination. The minimum and maximum (naked-eye) readouts of the colour change correspond to approximately 30 and 90 mJ cm−2, respectively, and they are achieved within seconds (Fig. 2b, black line). The latter indicates a fast switching of the spiropyran molecules in the modified paper, and the kinetics is comparable to the kinetics observed when the same polymer is immobilised on a planar, non-porous glass substrate.32
Fast switching is an obvious prerequisite for successful sensor development, however, as shown in the first line of Fig. 2a, the dynamic range of the sensing of UV light is limited. Hence, we followed a simple strategy to decelerate the chromatic response and extend the dynamic range of the colour change. We followed the hypothesis that the sensitivity of the photo-responsive material can be dynamically adjusted by controlling the photons that reach the spiropyran-modified surface. We used unmodified paper as a simple damping material because its porous and disperse medium significantly scatters incoming light.45 The spiropyran-modified paper was covered by additional paper sheets, and tight-contact illumination zones were defined (Scheme S3†). In a first trial, one or two pieces of conventional filter paper (Roth 15A) were used as the damping material. According to the digitalized images, the UV-induced colour change decelerates as the number of damping papers increases (Fig. S10†). Since commercial filter paper contains wet-strength agents, such as melamine-formaldehyde resin, which itself can absorb UV light, we decided to use self-made cotton-linters damping sheets of varying grammage. The damping process was carried out in the same manner as described above; however, in this case, we only used one sheet for damping with varying grammage. Fig. 2a (lines 2–6) shows photo-images of the spiropyran-functionalised paper under different damping conditions along with the corresponding grammage, thickness of the damping-sheet, and UV transmittance of the damping paper strips denoted on the left. As the paper grammage increases from 23 to 120 g m−2, the thickness increases from 58 to 211 μm, and the UV transmittance (measured separately) decreases from 40.1 to 8.5%. As expected, the colour change of the modified paper becomes less intense as the UV transmittance decreases when it is illuminated at the same UV dose, which indicates an increase in the dynamic range of sensing. The maximum UV dose for naked-eye readout of the spiropyran-functionalised paper is approximately 500, 1000, 1500, 2000 and more than 2000 mJ cm−2 for the damping paper with a grammage of 23, 32, 55, 79 and 120 g cm−2, respectively. To understand the colour change in more detail, the UV switching kinetics of spiropyran molecules was studied for the same samples. To quantify the kinetics, the obtained digitalized images were analysed as a grey value. Thus, the relationship between the grey value of spiropyran-functionalised paper and UV illumination time (which is equal to the UV dose divided by a constant UV flux of the UV chamber, 2.4 mW cm−2) under different damping conditions was evaluated (Fig. 2b). For the condition without damping (“no damping”), the mean grey value decreases as the UV illumination time increases and begins to level off at approximately 400 s (the relative UV dose is approximately 1000 mJ cm−2). According to the literature, the UV switching behaviour of the spiropyran molecules generally follows a first-order kinetics. Thus, we fitted our data using a simple exponential model (the straight lines in Fig. 2b). The slight deviation from first-order kinetics may be attributed to steric hindrance that occurs with an increase in the amount of UV-generated merocyanine molecules, which is consistent with the findings by other groups.30 As can be inferred from Fig. 2b, the grey value of spiropyran-functionalised paper increases when the damping paper grammage increases under exposure for the same UV illumination time (i.e., the same UV dose), which indicates that less MC molecules were generated. This phenomenon suggests that the damping method proposed that involves using lab-engineered paper sheets as ad-layers of varying grammage (i.e., different sheet thicknesses) is a simple yet very efficient way of extending the dynamic sensitivity of such spiropyran-functionalised paper-based sensors.
Finally, a first proof-of-concept application of this technique as a UV sensor was demonstrated using a colourimetric “strip-test” device (similar to the analysis of the pH value using pH strips). Here, the spiropyran-functionalised paper, covered by a damping paper, was illuminated with a certain UV dose (120 mJ cm−2). The colour of the illuminated paper strip was then compared with a photograph of standard colours that were obtained from the outlined experiments above. As shown in Fig. 3, the measured UV dose is highly consistent with the applied UV dose. The use of the spiropyran-functionalised paper as a UV sensor forms a highly sensitive and stable system owing to the covalent attachment of photochromic polymers. Additionally, the colourimetric readout requires negligible instrumental expense. Note, the back-switching process of the coloured paper in the dark has no significant impact for use as a colourimetric sensor (Fig. S11†). The material can be switched back by visible light, however, due to photooxidation processes, there is a distinct photofatigue which can interfere with the accuracy of the sensor (Fig. S12†), and thus a one-time use of such a paper-based device is favoured.46
In conclusion, a photochromic paper-based material was prepared by simple modification of paper substrates with spiropyran-containing polymers by dip-coating. Photo-cross-linkable benzophenone groups in the polymer were used to form polymer networks and to covalently attach the polymers to the cellulose fibres of the paper sheets. The amount of immobilised polymers on the paper substrates can be conveniently controlled by varying the polymer concentration during the dip-coating procedure. The dynamic range and sensitivity of the chromatic response of spiropyran molecules in the paper system can be controlled over a wide range by a simple-to-perform damping technique that involves using additional paper sheets of varying grammage to decrease the UV-transmittance. Because of its simple design and the fact that no additional equipment or energy source is required, this paper-based material can be used for the quantification of unknown UV doses in situations where no instrumental support is possible or desired.
Acknowledgements
We thank Martina Ewald and Heike Herbert for various technical support and PMV (TU Darmstadt) for the offer of paper sheets with a grammage of 23 g m−2. W. Li would like to thank the China Scholarship Council (CSC) for financial support. The Verband Deutscher Papierindustrie (VDP) and the Vereingung der Arbeitgeberverbände der Deutschen Papierindustrie (VAP) is thanked for financial support.Notes and references
- D. Roy, J. N. Cambre and B. S. Sumerlin, Prog. Polym. Sci., 2010, 35, 278 CrossRef CAS.
- F. Liu and M. W. Urban, Prog. Polym. Sci., 2010, 35, 3 CrossRef CAS.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101 CrossRef PubMed.
- (a) L. Dos Ramos, S. de Beer, M. A. Hempenius and G. J. Vancso, Langmuir, 2015, 31, 6343 CrossRef CAS PubMed; (b) X. Feng, H. Wu, X. Sui, M. A. Hempenius and G. J. Vancso, Eur. Polym. J., 2015, 72, 535 CrossRef CAS; (c) K. Zhang, X. Feng, X. Sui, M. A. Hempenius and G. J. Vancso, Angew. Chem., Int. Ed., 2014, 53, 13789 CrossRef CAS PubMed; (d) X. Feng, X. Sui, M. A. Hempenius and G. J. Vancso, J. Am. Chem. Soc., 2014, 136, 7865 CrossRef CAS PubMed.
- (a) V. Carias, J. Wang and R. Toomey, Langmuir, 2014, 30, 4105 CrossRef CAS PubMed; (b) O. O. Akintewe, S. J. DuPont, K. K. Elineni, M. C. Cross, R. G. Toomey and N. D. Gallant, Acta Biomater., 2015, 11, 96 CrossRef CAS PubMed; (c) M. C. Cross, R. G. Toomey and N. D. Gallant, Biomed. Mater., 2016, 11, 022002 CrossRef PubMed.
- I. Roy and M. N. Gupta, Chem. Biol., 2003, 10, 1161 CrossRef CAS PubMed.
- (a) N. Wagner and P. Theato, Polymer, 2014, 55, 3436 CrossRef CAS; (b) Y. Qui and K. Park, Adv. Drug Delivery Rev., 2001, 53, 321 CrossRef.
- M. Behland and A. Lendlein, Soft Matter, 2007, 3, 58 RSC.
- C. M. Lampert, Mater. Today, 2004, 7, 28 CrossRef CAS.
- V. I. Minkin, Chem. Rev., 2004, 104, 2751 CrossRef CAS PubMed.
- B. L. Feringa, R. A. van Delden, N. Koumura and E. M. Geertsema, Chem. Rev., 2000, 100, 1789 CrossRef CAS PubMed.
- F. M. Raymo and M. Tomasulo, Chem. Soc. Rev., 2005, 34, 327 RSC.
- J. A. Delaire and K. Nakatani, Chem. Rev., 2000, 100, 1817 CrossRef CAS PubMed.
- (a) D. Kessler, F. D. Jochum, J. Choi, K. Char and P. Theato, ACS Appl. Mater. Interfaces, 2011, 3, 124 CrossRef CAS PubMed; (b) A. Doron, E. Katz, G. Tao and I. Willner, Langmuir, 1997, 13, 1783 CrossRef CAS.
- F. M. Raymo, D. J. Williams, A. J. P. White and D. J. Williams, J. Org. Chem., 2003, 68, 4158 CrossRef CAS PubMed.
- X. Guo, D. Zhang, G. Zhang and D. Zhu, J. Phys. Chem. B, 2004, 108, 11942 CrossRef CAS.
- (a) R. J. Byrne, S. E. Stitzel and D. Diamond, J. Mater. Chem., 2006, 16, 1332 RSC; (b) A. Garcia, M. Marquez, T. Cai, R. Rosario, Z. Hu, D. Gust, M. Hayes, S. A. Vail and C. D. Park, Langmuir, 2007, 23, 224 CrossRef CAS PubMed.
- R. Klajn, Chem. Soc. Rev., 2014, 43, 148 RSC.
- P. K. Kundu, G. L. Olsen, V. Kiss and R. Klajn, Nat. Commun., 2014, 5, 3588 Search PubMed.
- D. Tobjörk and R. Österbacka, Adv. Mater., 2011, 23, 1935 CrossRef PubMed.
- J. Sarfraz, D. Tobjörk, R. Österbacka and M. Lindén, IEEE Sens. J., 2012, 12, 1973 CrossRef CAS.
- M. Xu, B. R. Bunes and L. Zang, ACS Appl. Mater. Interfaces, 2011, 3, 642 CAS.
- M. C. Barr, J. A. Rowehl, R. R. Lunt, J. Xu, A. Wang, C. M. Boyce, S. G. Im, V. Bulovic and K. K. Gleason, Adv. Mater., 2011, 23, 3500 CrossRef CAS PubMed.
- J. Y. Kim, S. H. Park, T. Jeong, M. J. Bae, S. Song, J. Lee, I. T. Han, D. Jung and S. Yu, IEEE Trans. Electron Devices, 2010, 57, 1470 CrossRef CAS.
- Y. H. Ngo, D. Li, G. P. Simon and G. Garnier, Adv. Colloid Interface Sci., 2011, 163, 23 CrossRef CAS PubMed.
- Q. Yan, J. Yuan, F. Zhang, X. Sui, X. Xie, Y. Yin, S. Wang and Y. Wei, Biomacromolecules, 2009, 10, 2033 CrossRef CAS PubMed.
- S. K. Mahadeva, K. Walus and B. Stoeber, ACS Appl. Mater. Interfaces, 2015, 7, 8345 CAS.
- W. Tian and J. Tian, Langmuir, 2014, 30, 3223 CrossRef CAS PubMed.
- A. Abdollahi, A. R. Mahdavian and H. Salehi-Mobarakeh, Langmuir, 2015, 31, 10672 CrossRef CAS PubMed.
- J. K. Rad and A. R. Mahdavian, J. Phys. Chem. C, 2016, 120, 9985 Search PubMed.
- A. Abdollahi, J. K. Rad and A. R. Mahdavian, Carbohydr. Polym., 2016, 150, 131 CrossRef CAS PubMed.
- H. Schenderlein, A. Voss, R. W. Stark and M. Biesalski, Langmuir, 2013, 29, 4525 CrossRef CAS PubMed.
- A. Boehm, M. Gattermayer, C. Trieb, S. Schabel, D. Fiedler, F. Miletzky and M. Biesalski, Cellulose, 2013, 20, 467 CrossRef CAS.
- A. Boehm, F. Carstens, C. Trieb, S. Schabel and M. Biesalski, Microfluid. Nanofluid., 2014, 16, 789 CrossRef CAS.
- M. Jocher, M. Gattermayer, H. J. Kleebe, S. Kleemann and M. Biesalski, Cellulose, 2015, 22, 581 CrossRef CAS.
- M. Janko, M. Jocher, A. Boehm, L. Babel, S. Bump, M. Biesalski, T. Meckel and R. W. Stark, Biomacromolecules, 2015, 16, 2179 CrossRef CAS PubMed.
- C. Bunte, O. Prucker, T. Koenig and J. Ruehe, Langmuir, 2010, 26, 6019 CrossRef CAS PubMed.
- M. Natali and S. Giordani, Chem. Soc. Rev., 2012, 41, 4010 RSC.
- A. Fihey, A. Perrier, W. R. Browne and D. Jacquemin, Chem. Soc. Rev., 2015, 44, 3719 RSC.
- G. Berkovic, V. Krongauz and V. Weiss, Chem. Rev., 2000, 100, 1741 CrossRef CAS PubMed.
- H. Görner and A. K. Chibisov, J. Chem. Soc., Faraday Trans., 1998, 94, 2557 RSC.
- Q. Chen, Y. Feng, D. Zhang, G. Zhang, Q. Fan, S. Sun and D. Zhu, Adv. Funct. Mater., 2010, 20, 36 CrossRef CAS.
- S. Liu, L. Tan, W. Hu, X. Li and Y. Chen, Mater. Lett., 2010, 64, 2427 CrossRef CAS.
- W. Hu, S. Liu, S. Chen and H. Wang, Cellulose, 2011, 18, 655 CrossRef CAS.
- A. K. Ellerbee, S. T. Phillips, A. C. Siegel, K. A. Mirica, A. W. Martinez, P. Striehl, N. Jain, M. Prentiss and G. M. Whitesides, Anal. Chem., 2009, 81, 8447 CrossRef CAS PubMed.
- G. Such, R. A. Evans, L. H. Yee and T. P. Davis, J. Macromol. Sci., Polym. Rev., 2003, 43, 547 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and compounds, instruments, experimental and analytical data. See DOI: 10.1039/c6ra23673a |
| This journal is © The Royal Society of Chemistry 2016 |