PDGF-BB, NGF and BDNF enhance pulp-like tissue regeneration via cell homing
Abstract
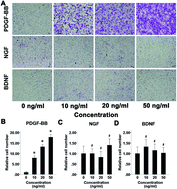
In this study, we investigated the cytobiological effects of platelet-derived growth factor BB (PDGF-BB), nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) on the culture of bone mesenchymal stem cells (BMSCs) from rats and explored a viable approach for regenerating ectopic dental pulp-like tissue via cell homing. In vitro, the proliferation, migration, and differentiation of rat BMSCs treated with different dosages of PDGF-BB, BDNF and NGF were evaluated using CCK-8, trans-well and quantitative real-time PCR (qRT-PCR) assays. In vivo, rats were randomly assigned to three groups: the control group, the low concentration group (L group) and the high concentration group (H group). The cytokines were delivered into endodontically treated human teeth, which were then implanted subcutaneously into the rat dorsum for 2 to 4 months. Next, a histologic analysis was employed to identify the regenerated dental pulp-like tissue. The results showed that PDGF-BB/NGF/BDNF facilitated BMSC proliferation in time- and dose-dependent manners. PDGF-BB significantly promoted the migration of BMSCs (P < 0.05). The maximum gene expressions of Runx2, osteopontin (OPN), markers of neural dendrites and somata-microtubule associated protein-2 (MAP2) and β-III-tubulin, were induced following treatment with 100 ng ml−1 PDGF-BB, NGF and BNDF respectively (P < 0.05). In vivo, well-vascularized pulp-like tissue was regenerated in both the H and L groups. This finding was confirmed via CD-34 immunohistological staining, which indicated newly formed vessels. More small vessels were observed in the H group than in the other groups (P < 0.05). In addition, positive signals for S-100 were only detected in the H group, which indicated newly formed nerve fibers. In conclusion, the current study provides evidence supporting the homing of endogenous mesenchymal stem cells via the combined use of a PDGF-BB/NGF/BDNF delivery system to regenerate ectopic pulp-like tissue.

 Please wait while we load your content...
Please wait while we load your content...