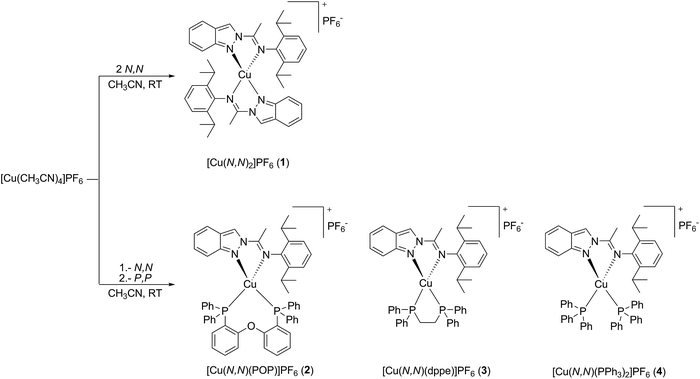
Synthesis of new phosphorescent imidoyl-indazol and phosphine mixed ligand Cu(I) complexes – structural characterization and photophysical properties†
Alan R. Cabrera*ae,
Ivan A. Gonzaleza,
Diego Cortés-Arriagadaa,
Mirco Natalib,
Heinz Berkec,
Constantin G. Daniliucd,
María B. Camaradae,
Alejandro Toro-Labbéa,
Rene S. Rojasa and
Cristian O. Salasa
aNucleus Millennium Chemical Processes and Catalysis, Faculty of Chemistry, Pontificia Universidad Católica de Chile, Casilla 306, Santiago-22, Chile. E-mail: arcabrer@uc.cl
bDepartment of Chemical and Pharmaceutical Sciences and Centro Interuniversitario per la Conversione Chimica dell'Energia Solare (sez. di Ferrara), University of Ferrara, Via Fossato di Mortara 17–19, 44121 Ferrara, Italy
cChemisches Institut, Universität Zürich, Winterthurer Strasse 190, CH-8057 Zürich, Switzerland
dOrganisch-Chemisches Institut, Universität Münster, Corrensstrasse 40, 48149 Münster, Germany
eUniversidad Bernardo O Higgins, Departamento de Ciencias Químicas y Biológicas, Laboratorio de Bionanotecnología, General Gana 1702, Santiago, Chile
First published on 5th January 2016
Abstract
Four mononuclear Cu(I) complexes were prepared, described as [Cu(N,N)2]PF6 (1) and [Cu(N,N)(P,P)]PF6 (2–4), where N,N is N-(1-(2H-indazol-2-yl)ethylidene)-2,6-diisopropylaniline and P,P are phosphine derived ancillary ligands (bis[2-(diphenylphosphino)phenyl]ether (POP), bis(diphenylphosphino)ethane (dppe) or 2 PPh3). These new species were characterized by NMR, FT-IR, elemental analyses, cyclic voltammetry, UV-Vis – emission spectroscopy, transient absorption spectroscopy and DFT calculations. In addition, complexes 1 and 2 were characterized by X-ray diffraction. The four complexes showed an MLCT absorption band between 400 and 450 nm, in addition to a weakly structured phosphorescence in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol
1 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol glassy matrix at 77 K. Complexes 2–4 have emission profiles that resemble the phosphorescence of the protonated N,N ligand, suggesting a triplet LC character of the lowest lying excited state at 77 K. By contrast, a mixed MLCT/LC triplet emission is most likely responsible for the phosphorescence in complex 1. Weak ligand-centered emission is also detected in the solid state at room temperature but only in the case of complexes 2 and 4, suggesting thermally activated deactivation processes in the case of 1 and 3. Notably, the transient absorption spectroscopy of complexes 2–4 in CH2Cl2 solution confirms a strong contribution from a ligand-centered (LC) triplet excited state, pointing towards a mixed 3MLCT/3LC character of the transient species in solution at room temperature, undergoing a non-radiative deactivation in the μs time-scale. This behavior markedly differs from that observed for complex 1, whose short-lived 3MLCT excited state is followed by ultrafast transient absorption spectroscopy.
methanol glassy matrix at 77 K. Complexes 2–4 have emission profiles that resemble the phosphorescence of the protonated N,N ligand, suggesting a triplet LC character of the lowest lying excited state at 77 K. By contrast, a mixed MLCT/LC triplet emission is most likely responsible for the phosphorescence in complex 1. Weak ligand-centered emission is also detected in the solid state at room temperature but only in the case of complexes 2 and 4, suggesting thermally activated deactivation processes in the case of 1 and 3. Notably, the transient absorption spectroscopy of complexes 2–4 in CH2Cl2 solution confirms a strong contribution from a ligand-centered (LC) triplet excited state, pointing towards a mixed 3MLCT/3LC character of the transient species in solution at room temperature, undergoing a non-radiative deactivation in the μs time-scale. This behavior markedly differs from that observed for complex 1, whose short-lived 3MLCT excited state is followed by ultrafast transient absorption spectroscopy.
Introduction
In recent years research on the development of optoelectronic devices has been intensified, mainly triggered by the enormous technological advances in areas such as flat displays and energy-efficient lighting.1 Transition metal complexes have played a leading role in the development of photochemical molecular devices, including nonlinear optics (NLO), organic light emitting diodes (OLED), and light emitting electrochemical cells (LEEC). All these systems require a specific and controlled response to an external energy input.2 To date, the most widely used devices are those based on second or third row transition metals, such as Ru, Re, Ir, Pt, and Os. However, these metals are of high cost and low natural abundance, generating an expensive large scale production costs of the emitting devices.3 One potential and viable solution is to use cheaper first-row transition metals, which have greater abundance. In this respect, emitting devices based on Cu(I) have attracted great attention, as copper is a metal of low toxicity and has been successfully tested in OLEDs.4The Cu(I) metal center has a 3d10 electronic configuration, and preferably form tetrahedral complexes. The work made by McMillin et al. established since the late 1970s the foundation of a chemistry with Cu(I) complexes bearing N,N phenanthroline ligands and their derivatives of the type [Cu(N,N)2]+. A basis was developed for the study of the relationship between molecular structure and photophysical properties of these complexes in solution.5 The absorption spectra of these types of complexes show bands in the UV region corresponding to LC (ligand centered) transitions, while in the visible region the spectra exhibit bands corresponding to MLCT (metal-to-ligand charge transfer) transitions, which are significantly influenced by the nature of the coordinated ligands. Due to the presence of a d10 closed shell and thus to the absence of d–d electronic transitions, these complexes exhibit triplet MLCT emission.6 The efficiency, however, is usually low in solution due to competitive non-radiative deactivations of the excited state accompanied by the structural reorganization of the complexes and/or the formation of exciplexes,7 whereas in the solid state it is expected that higher emission yields can be achieved precluding formation of exciplexes or dimeric aggregates, mainly because of a completed coordination sphere by coordination of a solvent molecule to the copper center.8 To overcome this issue the strategy adopted for [Cu(N,N)2]+ complexes was based on the use of highly sterically hindered N,N ligands which prevented (or at least minimized) the possibility of structural rearrangement and exciplex formation in the excited state.6,8 However, even in this case the low emission energies were usually responsible for the unexceptional photoluminescence properties.
Other strategies were applied to further understand the issues experienced when studying the heteroleptic complexes of the type [Cu(N,N)(P,P)]+, which contain bidentate phosphorus-based ligands replacing in the tetrahedral arranged homoleptic systems one N,N ligand.9 The use of phosphine ligands, which have a greater electron-withdrawing character than phenanthrolines,9l stabilizes the HOMO of the Cu(I) complex. In this case, the LUMO is still localized in the N,N ligand, therefore the P,P one is the responsible for the modulation of the MLCT energy which could result in an important effect over the emission efficiencies (according to the energy-gap law).6,9 On the other hand, the distinct coordination modes of different P,P ligands into the copper center may affect the energy of the MLCT emission resulting in color tunability via P,P substitution.9,10 Indeed, the choice of the phosphine ligand is crucial for the optimization of the photophysical properties of the heteroleptic Cu(I) compounds, which are influenced by the P–Cu–P bond angles, the size of the cone angle of monodentate phosphines, and the bite angle of the bidentate phosphines.10 Hence, the development of heteroleptic complexes proved to be an important and fundamental step forward in the aforementioned applications.
In this work four mononuclear Cu(I) complexes were synthesized and structurally characterized, one homoleptic of type [Cu(N,N)2]+, and three heteroleptic of type [Cu(N,N)(P,P)]+ (N,N = N-(1-(2H-indazol-2-yl)ethylidene)-2,6-diisopropylaniline). The isopropyl substituent exerts great steric hindrance, preventing structural rearrangement, and the indazole ring system is mainly responsible for the electronic delocalization in the ligand, favoring a charge transfer necessary for optoelectronic devices such as NLO, DSSC, OLED and LEEC.9,11 The P,P ligand systems are three different types of phosphines, namely bis[2-(diphenyl phosphino)phenyl]ether (POP), bis(diphenylphosphino)ethane (dppe) and triphenylphosphine (PPh3).
Results and discussion
Synthesis and structural characterization
The copper(I) complexes with the N,N ligand (N-(1-(2H-indazol-2-yl)ethylidene)-2,6-diisopropylaniline) and the P,P ancillary phosphine ligands (P,P = POP, dppe and PPh3) {[Cu(N,N)2]PF6 (1), [Cu(N,N)(POP)]PF6 (2), [Cu(N,N)(dppe)]PF6 (3) and [Cu(N,N)(PPh3)2]PF6 (4)}, were prepared according to literature methods, applying a severe control of the reaction stoichiometries (see Scheme 1).9a,12–14 The N,N ligand (N-(1-(2H-indazol-2-yl)ethylidene)-2,6-diisopropylaniline) was previously reported by our group.15 The homoleptic complex 1 was obtained in a straightforward synthesis by addition of [Cu(CH3CN)4]PF6 to a solution of 2 equivalents of the N,N ligand in acetonitrile. The heteroleptic species (2–4) were prepared in a two-step reaction via addition of one equivalent of the N,N ligand to a [Cu(CH3CN)4]PF6 solution in acetonitrile, followed by the addition of another equivalent of the phosphorus ligands (Scheme 1) furnishing high yields. Complexes 1–4 were air and thermally stable in solution as well as in the solid state. They were fully characterized by NMR, IR, elemental analyses and for complexes 1 and 2 in addition by X-ray diffraction analyses.Selected spectroscopic parameters of complexes 1–4 and of the N,N ligand are summarized in Table 1. The NMR spectra of the complexes are consistent with the formation of mononuclear pseudo-tetrahedral species coordinated by two bidentate ligands. In most cases, the 1H and 13C NMR spectra display low field shifts compared to the free N,N ligand due to the effect of coordination. The 31P NMR spectra of compounds 2–4 exhibit singlets at −12.0, 7.4 and 1.3 ppm, respectively, suggesting that the phosphorus ligands are symmetrically bounded to the metal centers comparable to previously reported Cu(I) complexes of related types.9d,10,16,17 In addition for the coordinated phosphine ligands, significant chemical shift changes are observed with respect to the free phosphine. The 19F and 31P NMR spectra of complexes 1–4 show similar chemical shifts for the PF6− counterion in CD2Cl2, indicating that these counterions are “free” ions and are not or to a very small extent only ion-pairing with the cationic complex parts (19F: −72.8 ppm (d); 31P: −144.3 ppm (sept)).
| N,N | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| a In CD2Cl2 solution. | |||||
| δ 1H NMR (ppm) | |||||
HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (indazole) N (indazole) |
8.98 | 8.80 | 9.06 | 9.06 | 9.22 |
| HCiPr | 2.74 | 2.79 | 2.59 | 2.81 | 2.53 |
CHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N3 N3 |
2.37 | 2.46 | 2.54 | 2.71 | 2.68 |
| CHiPr3 | 1.00 | 0.62 | 1.16 | 0.72 | |
| 0.34 | 0.49 | 0.80 | 0.37 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| δ 13C{H}NMR (ppm) | |||||
MeC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
154.7 | 157.4 | 157.3 | 160.3 | 158.0 |
| HCiPr | 28.8 | 28.9 | 29.4 | 29.1 | |
| CHiPr3 | 23.5 | 24.6 | 23.7 | 24.5 | |
| 22.9 | 22.9 | 23.4 | 23.2 | ||
CHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/i_char_e001.gif) N3 N3 |
16.5 | 16.1 | 17.9 | 15.8 | 18.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| δ 31P{H}NMR (ppm) | |||||
| PF6− | — | −144.3 | −144.3 | −144.4 | −144.3 |
| P,P | — | — | −12.0 | 7.4 | 1.3 |
The molecular structures of the Cu(I) complexes 1 and 2 were determined by X-ray diffraction studies and are shown in Fig. 1 and 2, respectively. In complex 1, the metal center has a distorted tetrahedral geometry as a consequence of the small bite angles of the two chelating N,N ligands [N3A–Cu1–N2A 79.1(2)°; N3B–Cu1–N2B 78.8(2)°] and a hindered substituent environment around the Cu(I) center, due to the isopropyl groups, which is corroborated by the dihedral angles [N1B–N2B–Cu1–N2A 112.1(3)°; N1B–N2B–Cu1–N3A −161.0(3)°]. On the other hand, complex 2 exhibits a less distorted tetrahedral geometry with a greater bite angle from the phosphine ligand [N2–Cu1–N3 77.0(2)°; P1–Cu1–P2 111.1(1)°]. The highly congested environment contributed by this ligand affects the Cu–N distances, lengthening from 2.055(4) Å (Cu1–N3A); 2.038(4) Å (Cu1–N2A) in complex 1 to 2.073(6) Å (Cu1–N2); 2.121(6) Å (Cu1–N3) in complex 2. In both complexes the diisopropylphenyl group is almost orthogonal to the plane formed by the indazole ring with a dihedral angle of 105.8(6)° (C12A–C11A–N3A–C8A) for complex 1 and 86.0(9)° (C12–C11–N3–C8) for complex 2, giving the metal center a large steric hindrance, specially the phosphine ancillary ligand in complex 2. For further information of bond distances and angles see the ESI.†
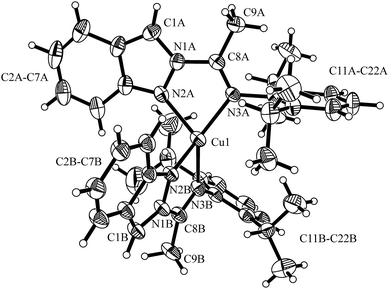 | ||
| Fig. 1 Molecular structure of [Cu(N,N)2]PF6 (1). Thermal ellipsoids are shown with 30% probability. PF6− counter ion was omitted for clarity. | ||
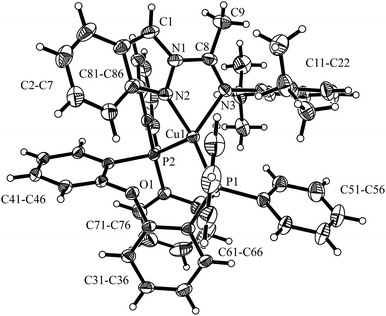 | ||
| Fig. 2 Molecular structure of [Cu(N,N)(POP)]PF6 (2). Thermal ellipsoids are shown with 30% probability. PF6− counter ion was omitted for clarity. | ||
UV-Vis absorption spectra
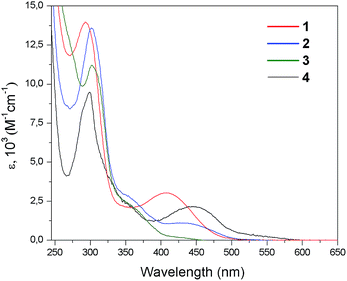
Fig. 3 shows the UV-Vis absorption spectra of the four Cu(I) complexes [Cu(N,N)2]PF6 (1), [Cu(N,N)(POP)]PF6 (2), [Cu(N,N)(dppe)]PF6 (3), and [Cu(N,N)(PPh3)2]PF6 (4) measured in CH2Cl2 solution at room temperature, while the absorption data are summarized in Table 2. The studied complexes 1–4 displayed electronic transitions similar to those of related Cu(I) complexes reported in the literature and their assignments were carried out accordingly.9d,g,h,k,n,17| Complex | Absorption | Emissiona | ||||
|---|---|---|---|---|---|---|
| CH2Cl2 λmax [nm] (εmax [M−1 cm−1]) | 77b K λmax [nm] | KBr pelletc | PMMA filmc | |||
| λmax [nm] | Φd | λmax [nm] | Φd | |||
| a In CH2Cl2 at room temperature the complexes were not emissive.b 4/1 ethanol/methanol glassy matrix excitation at 440 nm in 1, at 380 nm in 2–4.c Excitation at 400 nm in 2 and at 380 nm in 4.d Absolute quantum yields estimated using an integrating sphere set up. n.d. = not detected. | ||||||
| [Cu(N,N)2]PF6 (1) | 298 (9.5 × 103) | 580, 599, 632, 689 | n.d. | n.d. | n.d. | n.d. |
| 448 (2.1 × 103) | ||||||
| 500–550 (1 × 103) | ||||||
| [Cu(N,N)(POP)]PF6 (2) | 292 (1.4 × 104) | 603, 654, 714 | 625, 667 | 0.008 | 659 | 0.011 |
| 408 (3.0 × 103) | ||||||
| [Cu(N,N)(dppe)]PF6 (3) | 302 (1.4 × 104) | 616, 667, 724 | n.d. | n.d. | n.d. | n.d. |
| 438 (1.1 × 103) | ||||||
| [Cu(N,N)(PPh3)2]PF6 (4) | 309 (1.1 × 104) | 607, 658, 714 | 620, 657 | 0.004 | 650 | 0.008 |
| 430 (2.0 × 102) | ||||||
Complexes 1–4 exhibit one intense absorption band with maxima between 292 and 309 nm; these absorption bands where assigned to π → π* spin allowed transitions centered in the N,N ligand (LC) by means of comparison with the UV-Vis spectrum of the free and protonated N,N ligands (Fig. S1 of ESI†). At lower energies less intense and broad bands were observed, which were not found in the free ligand spectrum. In the case of complex [Cu(N,N)2]PF6 (1), this absorption appears with a maximum at 448 nm (ε = 2.1 × 103 M−1 cm−1) which can be assigned to a singlet metal-to-ligand charge transfer (1MLCT) transition [Cu(d) → N,N(π*)].5f,g,8 Furthermore, complex 1 shows a weak absorption in the range of 500–550 nm (ε ∼ 1 × 103 M−1 cm−1), which is not present in complexes 2–4. On the other hand, complexes 2–4 displays absorption bands at 408 nm (ε = 3 × 103 M−1 cm−1), 438 nm (ε = 1.1 × 103 M−1 cm−1), and 430 nm (ε = 0.2 × 103 M−1 cm−1), respectively. These absorption features are assigned to 1MLCT transitions as observed for Cu(I) complexes involving aromatic diimine ligands.9d,n These 1MLCT transitions mainly involve the 3d orbitals of the Cu(I) metal centers and the π* orbitals of the N,N ligands, specifically of the indazole fragment [Cu(d) → N,N(π*)], which are derived from the DFT calculations (vide infra).
The displacement of the 1MLCT band throughout the 1–4 series is in agreement with the reported behavior of Cu(I) complexes.8b,9d,18 Compared to complex 1, complexes 2–4 show a blue shift of their MLCT bands (between 10 and 40 nm). This effect is mainly related to the stabilization of the HOMO of the complexes via the interactions of the Cu(I) centers with the phosphine ancillary ligands, whereas in these cases the LUMO remains practically unchanged. The computed HOMO and LUMO energies and the HOMO–LUMO energy gaps (ΔE(H–L)) are sketched in Fig. 4. It is noted that the LUMO energies are almost the same for all complexes (∼−2.6 eV), in agreement with the small differences found among the electrochemical reduction potentials (see below Table 4). The latter is consistent with about 96% of the LUMO density delocalized on the N,N ligand for all the systems. On the other hand, the HOMO energy changes in a very sensitive way throughout the 1–4 series as expected on the basis of the different ligand environment (N,N vs. P,P ligands).
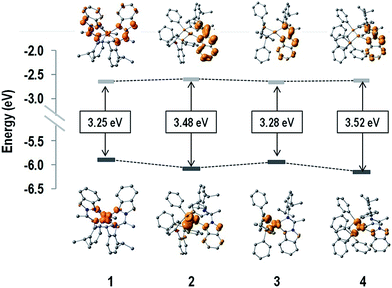 | ||
| Fig. 4 HOMO and LUMO density surfaces, and HOMO–LUMO energy gaps (ΔE(H–L)) of complexes 1–4. Hydrogen atoms were deleted for clarity. | ||
| Complex | State | Absorption λmax [nm] (E [eV]) | f | Monoexcitations (CI coef. and % cont.) | Description of the electronic transition |
|---|---|---|---|---|---|
| a E: transition energies, f: oscillator strength, CI: CI coefficient, % cont.: percentage of contribution to the excited state wave function, H: HOMO and L: LUMO. | |||||
| 1 | S1 | 513 (2.42) | 0.029 | H−1 → L (−0.28; 15%); H → L+1 (0.64; 81%) | Cu(d) + N,N(π) → N,N(π*); 1MLCT/1ILCT |
| S2 | 503 (2.46) | 0.009 | H−1 → L+1 (−0.35; 25%); H → L (0.60; 72%) | Cu(d) + N,N(π) → N,N(π*); 1MLCT/1ILCT | |
| S3 | 462 (2.68) | 0.111 | H−1 → L (0.63; 80%); H → L+1 (0.28; 16%) | Cu(d) + N,N(π) → N,N(π*); 1MLCT/1ILCT | |
| 2 | S1 | 427 (2.90) | 0.089 | H → L (0.70; 98%) | Cu(d) + P,P(π) → N,N(π*); 1MLCT/1LLCT |
| S4 | 352 (3.52) | 0.015 | H−3 → L (0.52; 55%); H−2 → L (−0.43; 37%) | N,N(π) + P,P(π) → N,N(π*); 1LLCT/1ILCT | |
| 3 | S1 | 458 (2.70) | 0.098 | H → L (0.71; 98%) | Cu(d) + P,P(π) → N,N(π*); 1MLCT/1LLCT |
| S2 | 414 (3.00) | 0.016 | H−1 → L (0.69; 96%) | Cu(d) + N,N(π) → N,N(π*); 1MLCT/1ILCT | |
| S3 | 358 (3.47) | 0.026 | H−2 → L (0.69; 96%) | N,N(π) → N,N(π*); 1ILCT | |
| 4 | S1 | 419 (2.96) | 0.082 | H → L (0.70; 97%) | Cu(d) + P,P(π) → N,N(π*); 1MLCT/1LLCT |
| S2 | 400 (3.10) | 0.014 | H−1 → L (0.67; 91%) | Cu(d) + N,N(π) → N,N(π*); 1MLCT/1ILCT | |
| S4 | 345 (3.59) | 0.020 | H−4 → L (−0.34; 24%); H−3 → L (0.59; 70%) | N,N(π) → N,N(π*); 1ILCT | |
| EOx [V] | ERed [V] | ΔE(Ox–Red) [V] | ΔE(H–L)b [eV] | |
|---|---|---|---|---|
| a Potential measured at room temperature, in CH2Cl2 solution at a scan rate of 50 mV s−1, versus Ag/AgCl electrode.b From DFT calculations, n.a. = not assigned. | ||||
| [Cu(N,N)2]PF6 (1) | 0.956 | n.a. | — | 3.25 |
| [Cu(N,N)(POP)]PF6 (2) | 1.512 | −1.107 | 2.619 | 3.48 |
| [Cu(N,N)(dppe)]PF6 (3) | 1.239 | −1.138 | 2.377 | 3.28 |
| [Cu(N,N)(PPh3)2]PF6 (4) | 1.337 | −1.231 | 2.568 | 3.52 |
In particular, substitution of N,N ligand with a P,P ligand causes stabilization of the HOMO level, thus determining an increase of the ΔE(H–L) values on going from the homoleptic complex 1 to the heteroleptic complexes 2–4, which is in agreement with the electrochemical gaps (see below Table 4). Moreover, as for [Cu(N,N)(P,P)]PF6 complexes, the spatial arrangement of the phosphines around the metal center (bite angle, geometric constraint, and steric hindrance)9a,14c and, in the case of complex 4, the rotation of the PPh3 affects both the energy of the MLCT band as well as its intensity (ε). In particular, wider P–Cu–P bite angles should increase the MLCT absorption energy as a consequence of the variation in the d–σ* interactions.9a,d Indeed, this consideration can explain the lowest MLCT energy observed for complex 3 with respect to complexes 2 and 4 (Fig. 3 and Table 2), which correlates with the smallest P–Cu–P angle within the heteroleptic 2–4 series (111.1(3)° for complex 2 as obtained from X-ray analyses, 88.65° for complex3 and 119.83° for complex 4 as obtained from DFT computations, see ESI†). This is also consistent with the smaller HOMO–LUMO gap estimated via DFT calculations as well as with the smaller electrochemical gap (see below) observed for 3 with respect to complexes 2 and 4. In addition, the increase in the geometric constraint of the phosphine is observed to influence the intensity of the MLCT absorption, with the molar absorptivity showing a progressive enhancement in the order PPh3 < dppe < POP.
To gain further insight into the low-energy transitions of complexes 1–4, the low-lying excited states were computed by means of time dependent DFT calculations (TD-DFT). Solvent effects were included by the PCM method19 with CH2Cl2 as solvent. Table 3 summarizes the transition energies, single excitations (and contributions) and nature of the low-lying excited states of complexes 1–4, and Fig. 5 depicts the hole and electron distributions.
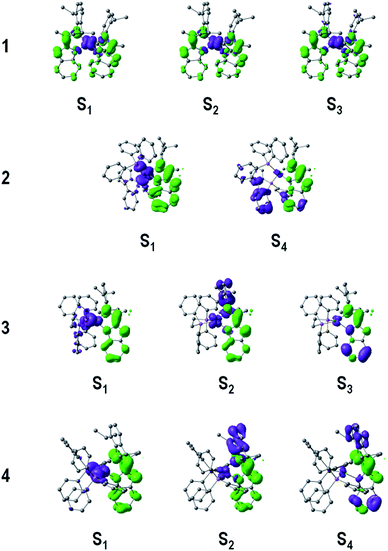 | ||
| Fig. 5 Hole (purple) and electron (green) distributions of selected singlet excited states (Si) of complexes 1–4. Hydrogen atoms were deleted for clarity. | ||
In complex 1 the computed transition at 462 nm (f = 0.111) could be related to the intense experimental absorption band at lower energies, which results from an electron promotion from HOMO−1 → LUMO (0.63), and a small contribution from the HOMO → LUMO+1 (0.28) transition. Note that the HOMO (and HOMO−1) is mainly composed of 53% 3d orbital character from the metal core. In addition, significant contributions to HOMO (HOMO−1) from the N,N ligands are observed stemming from the p orbitals of the nitrogen atoms involved in the Cu–N bonds and the carbon atoms in the aniline rings, with values of 28% (34%) and 11% (18%) orbital contributions, respectively. In addition, LUMO and LUMO+1 are delocalized in the indazole moieties of the N,N ligands by ∼88%. Therefore, the excited state at 462 nm is mainly assigned to a metal-to-ligand charge transfer (1MLCT) transition [Cu(d) → N,N(π*)] with some intra-ligand charge-transfer character (1ILCT) [N,N(π) → N,N(π*)], which is consistent with the hole–electron distribution shown in Fig. 5. Furthermore, poorly intense transitions at 513 (f = 0.029) and 503 nm (f = 0.01) display the same 1MLCT/1ILCT character, and are assigned to the weak band of the experimental spectrum falling in the range of 500–550 nm for complex 1.
The heteroleptic complexes 2–4 exhibit the HOMO−n → LUMO transitions in the range of 350–450 nm, where the LUMO is mainly consisting of π* orbitals of the indazole part of the N,N ligand. The first singlet excited state of complex 2 appears at 427 nm (f = 0.09), in good agreement with the experimental data; this HOMO → LUMO transition displays a mixed 1MLCT and 1LLCT character [Cu(d) + POP(π) → N,N(π*)], since the HOMO of complex 2 is localized mainly on the metal center (31%) and on the P,P ligand (POP) (48%).
Unlike for systems 1 and 2, the experimental UV-Vis spectrum of complex 3 displays a shoulder structure between 350 and 500 nm and the TD-DFT calculations confirms this interpretation. The band at lower energies (458 nm, f = 0.098) is due to a HOMO → LUMO transition with a mixed 1MLCT/1LLCT character [Cu(d) + dppe(π) → N,N(π*)]. This excited state involves an electron transition from the metal center and the P,P ligand (dppe) [where the HOMO resides: Cu (32%) and dppe (54%)] to the indazole part of the N,N ligand. In addition, a S2 excited state of 1MLCT/1ILCT character [Cu(d) + N,N(π) → N,N(π*)] is computed at 414 nm (f = 0.016), which shows a smaller oscillator strength compared to the S1 state explaining the broad low-intensity experimental band between 400 and 450 nm. In addition, a 1ILCT (π → π*) transition (centered in the indazole part of the N,N ligand) is found at higher energies for complex 3 causing the shoulder at ∼358 nm.
Finally, complex 4 revealed similar absorption band features as complex 3 in the range of 350–400 nm, but with lower ε values, which was attributed to the high degree of freedom and vibrational character of the PPh3 groups in solution. The latter is consistent with the S1 state (HOMO → LUMO) computed at 419 nm (f = 0.082), where the HOMO is mainly localized at the P,P ligand (48%) and in part at the metal center (28%), thus this transition is expected to be observed with a low intensity due to the rotational character of the PPh3 ligands. Moreover, the S2 and S4 states show the same electronic structure as for 3, which posses a larger ligand-centered character involving mainly the N,N ligand (>67%).
Electrochemical properties
The electrochemical properties of the complexes were determined by cyclic voltammetry in dichloromethane solution and the data are summarized in Table 4. Complex 1 shows the lowest oxidation potential (0.956 V) of all complexes. This oxidation is associated to the irreversible Cu(II)/Cu(I) processes.9h,20 Unfortunately, the reduction processes of complex 1 could not be assigned due to a poorly resolved shoulder peak. Presumably, this broad reduction peak likely involves multiple electron-transfer and a fast kinetic ligand reduction.21Complex 2 exhibits the higher oxidation potential (1.512 V) within the 1–4 series, revealing the better π acceptor character of the POP ligand over the N,N ligand. For complexes 3 and 4, irreversible anodic processes were observed at potentials of 1.239 V and 1.337 V, respectively, associated to the Cu(II)/Cu(I) couple; this difference is mainly due to the effect of the different P,P ligands on the metal center. On the other hand, the cathodic processes observed for complexes 2–4 fall at similar potential values, in agreement with the similar LUMO energies obtained from DFT calculations (see Fig. 4). Also, these results are consistent with the MLCT energy values observed in the absorption spectra of the complexes. For instance, complexes 2 and 4 exhibit the highest ΔE(Ox–Red) and ΔE(H–L), thus they show the greatest blue shift in the absorptions spectrum.
Luminescence properties
At room temperature in deaerated CH2Cl2 solution all the four complexes are not emissive. Therefore, the luminescent properties of all complexes were studied in the solid state at 77 K (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol
1 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol glassy matrix), in KBr pellet, and in a PMMA film.22,23 The results are depicted in Fig. 6 and the data summarized in Table 2.
methanol glassy matrix), in KBr pellet, and in a PMMA film.22,23 The results are depicted in Fig. 6 and the data summarized in Table 2.
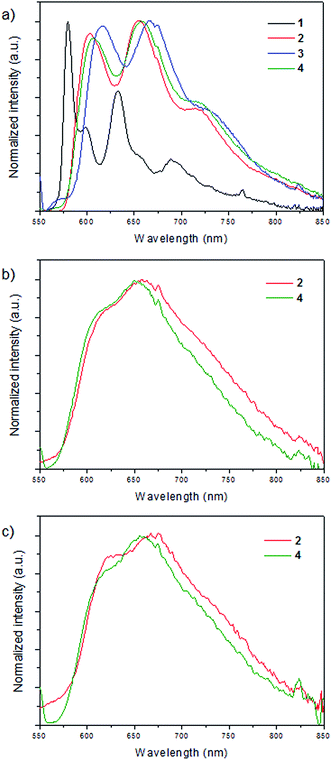
In the rigid matrix at 77 K, complex 1 displays a structured emission with relative maxima at 580, 599, 632 nm, which are considerably blue-shifted when compared to the related emissions of complexes 2–4, the luminescence of which has a structured profile featuring three different maxima (at 603, 654, 714 nm for 2, at 616, 667, 624 nm for 3, and 607, 658, 714 nm for 4, see Table 2), thus suggesting a similar character of the emitting state throughout the 2–4 series. Interestingly, the spectra of complexes 2–4 shown in Fig. 6a resemble the phosphorescence spectrum of the protonated N,N ligand (Fig. S2 of the ESI†). This clearly means that for 2–4 the emitting state at 77 K is mainly of ligand-centered (LC) character, whereas in the case of 1 a mixed MLCT/LC emission is most likely responsible for the differences in the structure of the luminescence profiles. The appreciable blue-shift of the emission of 1 with respect to those of 2–4 is also consistent with this notion. These findings can be rationalized considering the destabilization of the triplet MLCT excited state with respect to the ligand-centered triplet excited state occurring in the 77 K rigid medium. This destabilization is considerably larger in the case of the heteroleptic compounds 2–4 rather than for the homoleptic complex 1 as a result of the electronic effects exerted by the phosphine-based ligands.
In the solid state at room temperature, both in a PMMA film and a KBr pellet, emission is detected only for compounds 2 and 4 (Fig. 6b and c) with quantum yields between 0.004 and 0.011 (see Table 2). Complexes 1 and 3 display negligible photoluminescence. The emission profile for both complexes and in both matrices features a band with maximum at ca. 650 nm and a shoulder in the blue which is comparable in terms of both shape and energy with the structured emission observed in the glassy matrix at 77 K. This suggests that emission at room temperature of complexes 2 and 4 is mainly of ligand-centered (3LC) character, in agreement with the destabilization of the MLCT excited state that take place in the solid state.
The most likely explanation for the negligible emission originating from the excitation of complexes 1 and 3, either in PMMA film or KBr pellet, is that thermal population of the closely-lying 3MLCT excited state may take place at room temperature and non-radiative deactivation may become more favorable with respect to the 77 K glassy matrix (where an MLCT contribution is indeed observed in the emission of 1). This is correlated to the lower energies of the 3MLCT state in compounds 1 (homoleptic complex) and 3 (due to the smallest P–Cu–P angle within the heteroleptic complexes) with respect to complexes 2 and 4, consistently with both the absorption and electrochemical data as well as with the theoretical calculations.
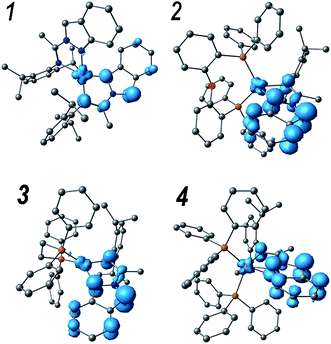
DFT calculations of the first triplet excited states (T1) (at its optimized geometries) confirmed the nature of the emissive states. Fig. 7 shows the T1 spin densities of compounds 1–4. Note that in the triplet electronic states, two unpaired electrons are found and the spin densities sum up to the total of unpaired electrons. In the case of complex 1, the unpaired electrons are mainly localized on the N,N ligand (1.61e) with a minor contribution from the metal core (0.39e) indicating that the nature of the T1 transition is ∼80% of 3LC, while the 3MLCT contribution is only about 20%, which is in agreement with the experimental findings. On the other hand, in compounds 2–4 the metal contributions of the T1 states are only ∼10%, clearly indicating that their emissive states are mainly involving the 3LC states as explained from the experimental results. Then the low 3MLCT contributions decrease the spin orbit coupling and consequently the luminescence efficiency. Specifically, the spin densities in the metal core appear to be 0.12, 0.20 and 0.30e for compounds 2, 3 and 4, respectively, while in the N,N ligands values of 1.75, 1.60 and 1.74e are found, respectively.
Transient absorption spectroscopy
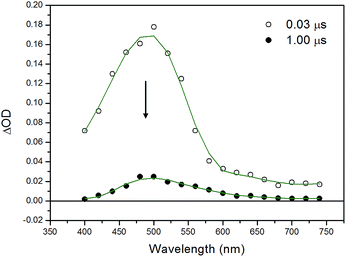
In order to acquire deeper understanding of the photophysical properties, transient absorption spectroscopy studies were performed on complexes 1–4. Laser flash photolysis studies have been first applied to the whole series of complexes spanning a time window from ns to ms. Whereas the homoleptic complex 1 does not display any transient signal upon 355 nm excitation in CH2Cl2 solution (vide infra), the heteroleptic complexes 2–4 show similar transient signatures. Going into more detail, a transient species is immediately observed after the laser pulse in the case of complex 2. Its spectrum (Fig. 8) features an absorption centered at 490 nm with a tail at longer wavelengths. This spectrum decays monotonically to the baseline with oxygen-dependent kinetics (τ = 760 ns under aerated conditions, Fig. S3 of ESI,† and τ = 19.9 μs under degassed conditions, Fig. S4 of ESI†) clearly confirming the triplet character of the transient species. The presence of an apparent bleaching at ∼400 nm is consistent with the depletion of the MLCT absorption in the excited state. However, the considerably long lifetime in the absence of any detectable emission at room temperature, clearly suggests a strong contribution from a ligand-centered triplet excited state (3LC) consistent with the photoluminescence data. Therefore these observations point towards a mixed 3MLCT/3LC character of the transient species in 2 undergoing non-radiative deactivation in the μs time-scale.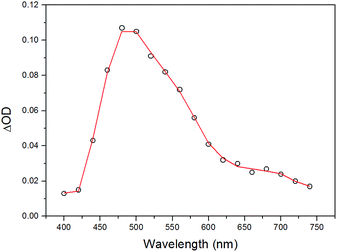 | ||
| Fig. 8 Transient absorption spectrum of complex 2 in CH2Cl2 obtained by laser flash photolysis (excitation at 355 nm, FWHM = 6–8 ns) at 20 ns time delay. | ||
As far as complex 3 is concerned, the transient spectrum detected in the ns time-scale is depicted in Fig. 9. It displays a broad absorption centered at ca. 480 nm with a tail at longer wavelengths, very similar to the ones observed for the remaining heteroleptic complexes (Fig. 8 for 2 and see below Fig. 10 for 4), thus suggesting a similar character of the transient species. On the other hand, an interesting difference is observed as far as the kinetics of the transient absorption decay is concerned. The transient species indeed decays with oxygen dependent kinetics with a lifetime of τ = 540 ns under air-equilibrated conditions (Fig. S5 of ESI†) and τ = 960 ns after oxygen removal (Fig. S6 of ESI†). The elongation of the lifetime is consistent with the triplet character of the deactivating excited state. However, the small entity of the lifetime elongation (compared with what observed for complexes 2 and 4, see below) may suggest that the degree of 3LC/3MLCT mixing is lower and the deactivating species most likely displays a larger 3MLCT character than in the case of complexes 2 and 4. In this situation, the higher contribution of the metal center in the excited state would increase the spin–orbit coupling thus fostering a faster excited state decay. The reasons for this different behavior may lie on the stereoelectronic effect exerted by the dppe ancillary ligand which, as stated above, forces a smaller P–Cu–P angle with respect to the POP and PPh3 ligands, thus lowering the MLCT energy.
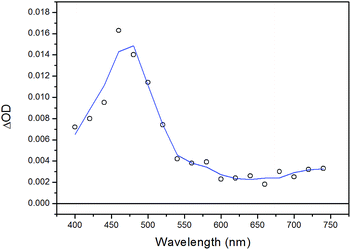 | ||
| Fig. 9 Transient absorption spectra of complex 3 in CH2Cl2 obtained by laser flash photolysis (excitation at 355 nm, FWHM = 6–8 ns) at 30 ns time delay. | ||
Regarding complex 4, the excited state dynamics is biphasic in nature. Indeed, the transient spectrum, which is detected immediately after the laser pulse (Fig. 10, black trace), featuring an intense absorption with a maximum at 500 nm with a tail at longer wavelengths, is observed to undergo a fast decay (time-constant of τ = 91 ns, Fig. S7 of ESI†) yielding a transient spectrum with similar spectral characteristics (Fig. 10, red trace), only differing from the former in the presence of a more intense bleaching at λ < 450 nm and a more accentuated absorption tail at λ > 550 nm (see the comparison of the normalized spectra in Fig. S8 of ESI†). This transient signal then decays to the baseline with oxygen-dependent kinetics (τ = 635 ns under aerated conditions, Fig. S7 of ESI,† τ = 37.7 μs under degassed conditions, see Fig. S9 of ESI†), supporting the triplet nature of the deactivating excited state. Similarly to complex 2, the spectral shape of the deactivating transient signal, as well as the observed long lifetime strongly suggests a mixed 3MLCT/3LC character of the transient species in 4. A slow thermal equilibration between the 3MLCT and the 3LC excited states,23 or a competitive parallel deactivation from a different, closely-lying excited state level may account for the faster decaying component.‡
As previously mentioned, no transient processes have been observed in the ns time-scale, as far as the homoleptic complex 1 is concerned. Therefore, in order to clarify the excited state behavior of 1 ultrafast transient absorption spectroscopy studies have been undertaken. The excited state dynamics is shown in Fig. 11 and can be split into two different processes. The first process (Fig. 11a) involves the evolution of a structured spectrum (sampled at a time-delay τ = 1 ps) with three distinct maxima at 520, 555, and 615 nm to a featureless spectrum with a broad absorption (maximum at 560 nm) and takes place with a time-constant of τ = ca. 2 ps (Fig. S10 of ESI†). The second process (Fig. 11b) is defined by the monotonic decay of this broad transient spectrum to the baseline and occurs with a time-constant of τ = ca. 280 ps (Fig. S10 of ESI†). The prompt transient spectrum with its multi-peak characteristic can be very likely attributed to the localized singlet MLCT excited state, stabilized after sub-picosecond flattening distortion.24 The presence of an apparent bleaching at λ < 480 nm is consistent with the MLCT depletion in the excited state. As a consequence, the first process (τ = ca. 2 ps) can be ascribed to the fast singlet-triplet intersystem crossing, mainly dictated by the spin–orbit coupling promoted by the heavy copper center (MLCT bleaching is indeed still retained after the fast process). Finally, the second process (τ = ca. 280 ps) corresponds to the decay of the triplet MLCT excited state to the ground state.
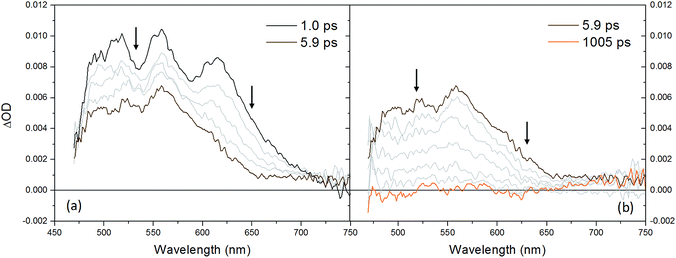 | ||
| Fig. 11 Transient absorption spectra obtained by ultrafast spectroscopy (excitation wavelength λ = 400 nm) of complex 1 in CH2Cl2 between (a) 1.0–5.9 ps and (b) 5.9–1005 ps. | ||
In general, the observed different lifetimes (e.g., the smaller one measured in 1 with respect to the remaining complexes 2–4 and the slight difference observed in 3 with respect to complexes 2 and 4) are intimately connected to the different character of the lowest lying excited state, namely pure 3MLCT and mixed 3LC/3MLCT, and the degree of mixing between the latters, which is determined by the different electronic effects played by the presence or absence of the ancillary P,P ligand, as well as their steric constraint.
Conclusions
A series of new mixed ligands Cu(I) complexes (1–4) were prepared via a simple synthetic route of ligand exchanges with high yields. The X-ray single crystal characterization of complexes 1 and 2 showed that the Cu(I) metal centers have a pseudo-tetrahedral geometries with a highly congested environment. The photophysical properties of complexes 1–4 were also successfully investigated. The four complexes exhibited MLCT absorption bands in the UV-Vis spectra in the range 400–450 nm, with energies and intensities dependent on the type of coordinated ligand. At room temperature the four weakly luminescent in a glassy matrix at 77 K. In the glassy matrix, complex 1 displayed a structured emission, which was considerably blue-shifted when compared to the emission of complexes 2–4. The luminescence of the latter was similar and resembled the luminescence of the protonated N,N ligand thus indicating a large contribution from a 3LC excited state.The negligible emissions of complexes 1 and 3, at room temperature in the solid state (PMMA film or KBr pellet), suggest thermally activated deactivation processes instead of complexes 2 and 4 that show a weak ligand-centered emission with quantum yields between 0.004 and 0.011.
Furthermore, transient absorption spectroscopy studies were exploited to follow the decay of the lowest lying excited states at room temperature. While complex 1 displayed a fast decay of the 3MLCT excited state to the ground state occurring with a lifetime of τ = ca. 280 ps, complexes 2–4 showed considerably long lifetimes in the μs time scale, which in the absence of any detectable emission, clearly suggested a mixed 3MLCT/3LC character of the transient species with a strong contribution arising from a ligand-centered (LC) triplet excited state, consistent with the photoluminescence data at 77 K.
Although complexes 1–4 are not emissive in CH2Cl2 solution, the observed emission in a rigid medium shows us that complex 2, bearing POP phosphine ancillary ligand, has the better structural and photophysical properties. In conclusion, based on the deep photophysical study performed, we can deduce that Cu(I) heteroleptic complexes with N,N imidoyl-indazole derivative ligands and POP phosphine can be potentially exploited as cheap materials for LEEC devices. Further efforts in the research towards the synthesis and applications of Cu(I) heteroleptic complexes, with small changes in the molecular structure of the N,N ligand and using the POP phosphine, are necessary for the improvement of the observed properties and these are currently ongoing in our laboratory.
Experimental
General remarks
All reagents were purchased from Sigma-Aldrich and used as received, unless otherwise specified. N,N ligand was prepared as described in the literature.15 NMR spectra were recorded on NMR Bruker AV 400. Chemical shifts are given in parts per million relative to TMS [1H and 13C, δ(SiMe4) = 0] or an external standard [δ(BF3OEt2) = 0 for 11B NMR, δ(CFCl3) = 0 for 19F NMR]. Most NMR assignments were supported by additional 2D experiments. Elemental analysis data were recorded on a Foss-Heraeus CHNO-Rapid analyzer. For Mass spectra were obtained on an HP 5988A spectrometer (Hewlett-Packard, Palo Alto, CA, USA). FT-IR spectra were recorded on a Bruker Vector-22 Spectrophotometer using KBr pellets. Electrochemical measurements were performed at a CHI730E potentiostat in a conventional three-compartment, three-electrode cell. Thin layer chromatography (TLC) was performed using Merck GF-254 type 60 silica gel. Column chromatography was carried out using Merck silica gel 60 (70–230 mesh). UV-Vis absorption spectra were recorded on a Jasco V-570 UV/Vis/NIR spectrophotometer. Photoluminescence spectra were taken on an Edinburgh Instrument spectrofluorimeter. Dichloromethane solutions of the complexes were previously degassed with N2 for approximately 20 min. KBr pellets containing complexes 1–4 were prepared by grinding the solid sample with solid potassium bromide purchased from Sigma-Aldrich. Poly(methyl methacrylate) (PMMA) films were prepared from CH2Cl2 solutions of 1–4 and low molecular weight poly(methyl methacrylate) followed by drop casting onto a glass surface. In both cases the concentration was adjusted depending on the absorption coefficient of the sample. The thickness of the polymeric film was not controlled. Femtosecond time-resolved experiments were performed using a pump–probe setup based on the Spectra-Physics Hurricane Ti:sapphire laser source and the Ultrafast Systems Helios spectrometer. The time-resolved spectral data were analyzed with the Ultrafast Systems Surface Explorer Pro software. Nanosecond transient measurements were performed with a custom laser spectrometer comprised of a Continuum Surelite II Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YAG laser (FWHM = 6–8 ns). For X-ray crystal structure analysis, data sets were collected with a Nonius Kappa CCD diffractometer; full details can be found in the independently deposited crystallography information files (cif), CCDC numbers: 1422058 for compound 1 and 1422059 for compound 2.†
YAG laser (FWHM = 6–8 ns). For X-ray crystal structure analysis, data sets were collected with a Nonius Kappa CCD diffractometer; full details can be found in the independently deposited crystallography information files (cif), CCDC numbers: 1422058 for compound 1 and 1422059 for compound 2.†
DFT calculations
DFT calculations were performed in the Gaussian 09 program with the B3LYP25 functional. The 6-31G(d,p) basis set was used for H, C, N, O and F atoms; the quasi-relativistic pseudopotential and basis set LANL2DZ26 was adopted for the metal. Ground states were fully optimized without symmetry restrictions, and vibrational frequencies were obtained in order to insure that optimized structures correspond to energy minimum, obtaining only positive frequencies. At the TD-DFT methodology, the first fifty singlet excited states were obtained to account for the characteristics of the experimental UV-Vis spectrum. Solvent effects were included by the PCM method,19,27 with dichloromethane as solvent. Hole–electron distributions and molecular orbital compositions were obtained by the Multiwfn code.28Synthesis of complexes
Acknowledgements
Financial support from FONDECYT projects No. 1130077 and Millennium Science Initiative (ICM, Chile) for support through the Millennium Nucleus of Chemical Processes and Catalysis (CPC), project NC120082. A. C. is grateful for a postdoctoral fellowship, FONDECYT 3140425. I. G. is grateful for a postdoctoral fellowship, FONDECYT 3160285. H. B. gratefully acknowledges support from the University of Zurich, Switzerland. M. N. gratefully acknowledges FIRB RBAP11C58Y ‘‘NanoSolar’’ for funding.Notes and references
- (a) F. So, J. Kido and P. Burrows, MRS Bull., 2008, 33, 663 CrossRef CAS; (b) Y. Chi and P.-T. Chou, Chem. Soc. Rev., 2010, 39, 638 RSC.
- (a) Y.-L. Loo and J. McCulloch, MRS Bull., 2008, 33, 653 CrossRef CAS; (b) U. Mitschke and P. J. Bäuerle, Mater. Chem., 2000, 10, 1471 RSC; (c) H. Yersin, Highly Efficient OLEDs with Phosphorescent Materials, Wiley-VCH, Weinheim, Germany, 2008 Search PubMed.
- (a) Y. You and S. O. Park, Dalton Trans., 2009, 1267 RSC; (b) E. Holder, B. M. W. Langeveld and U. S. Schubert, Adv. Mater., 2005, 17, 1109 CrossRef CAS; (c) L. He, J. Qiao, L. Duan, G. Dong, D. Zhang, L. Wang and Y. Qiu, Adv. Funct. Mater., 2009, 19, 2950 CrossRef CAS; (d) T. Tsuzuki and S. Tokito, Adv. Mater., 2007, 19, 276 CrossRef CAS; (e) C.-H. Yang, Y.-M. Cheng, Y. Chi, C.-J. Hsu, F.-C. Fang, K.-T. Wong, P.-T. Chou, C.-H. Chang, M.-H. Tsai and C.-C. Wu, Angew. Chem., Int. Ed., 2007, 46, 2418 CrossRef CAS PubMed; (f) C. Ulbricht, B. Beyer, C. Friebe, A. Winter and U. S. Schubert, Adv. Mater., 2009, 21, 4418 CrossRef CAS; (g) L. Flamigni, A. Barbieri, C. Sabatini, B. Ventura and F. Barigelletti, Top. Curr. Chem., 2007, 281, 143 CrossRef CAS; (h) C.-M. Che, C.-C. Kwok, S.-W. Lai, A. F. Rausch, W. J. Finkenzeller, N. Zhu and H. Yersin, Chem.–Eur. J., 2010, 16, 233 CrossRef CAS PubMed; (i) J. A. G. Williams, Top. Curr. Chem., 2007, 281, 205 CrossRef CAS; (j) J. A. G. Williams, S. Develay, D. L. Rochester and L. Murphy, Coord. Chem. Rev., 2008, 252, 2596 CrossRef; (k) J. Kalinowski, V. Fattori, M. Cocchi and J. A. G. Williams, Coord. Chem. Rev., 2011, 255, 2401 CrossRef CAS; (l) P.-T. Chou and Y. Chi, Eur. J. Inorg. Chem., 2006, 3319 CrossRef CAS; (m) J. Breu, C. Kratzer and H. Yersin, J. Am. Chem. Soc., 2000, 122, 2548 CrossRef CAS.
- (a) M. G. Crestani, G. F. Manbeck, W. W. Brennessel, T. M. McCormick and R. Eisenberg, Inorg. Chem., 2011, 50, 7172 CrossRef CAS PubMed; (b) M. Hashimoto, S. Igawa, M. Yashima, I. Kawata, M. Hoshino and M. Osawa, J. Am. Chem. Soc., 2011, 133, 10348 CrossRef CAS PubMed; (c) A. Barbieri, G. Accorsi and N. Armaroli, Chem. Commun., 2008, 19, 2185 RSC; (d) C.-W. Hsu, C.-C. Lin, M.-W. Chung, Y. Chi, G.-H. Lee, P.-T. Chou, C.-H. Chang and P.-Y. Chen, J. Am. Chem. Soc., 2011, 133, 12085 CrossRef CAS PubMed; (e) R. Czerwieniec, J. Yu and H. Yersin, Inorg. Chem., 2012, 51, 1975 CrossRef CAS; (f) H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, Coord. Chem. Rev., 2011, 255, 2622 CrossRef CAS; (g) R. Czerwieniec, T. Hofbeck, O. Crespo, A. M. Laguna, M. Concepcion-Gimeno and H. Yersin, Inorg. Chem., 2010, 49, 3764 CrossRef CAS PubMed; (h) K. A. Barakat, T. R. Cundari and M. A. Omary, J. Am. Chem. Soc., 2003, 125, 14228 CrossRef CAS PubMed; (i) S. Igawa, M. Hashimoto, I. Kawata, M. Hoshino and M. Osawa, Inorg. Chem., 2012, 51, 5805 CrossRef CAS PubMed; (j) D. M. Zink, M. Bächle, T. Baumann, M. Nieger, M. Kühn, C. Wang, W. Klopper, U. Monkowius, T. Hofbeck, H. Yersin and S. Bräse, Inorg. Chem., 2013, 52, 2292 CrossRef CAS PubMed; (k) D. M. Zink, T. Baumann, M. Nieger, E. C. Barnes, W. Klopper and S. Bräse, Organometallics, 2011, 30, 3275 CrossRef CAS; (l) L. Zhang, B. Li and Z. Su, J. Phys. Chem. C, 2009, 113, 13968 CrossRef CAS; (m) A. Tsuboyama, K. Kuge, M. Furugori, S. Okada, M. Hoshino and K. Ueno, Inorg. Chem., 2007, 46, 1992 CrossRef PubMed; (n) G. F. Manbeck, W. W. Brennessel and R. Eisenberg, Inorg. Chem., 2011, 50, 3431 CrossRef CAS PubMed; (o) L. Bergmann, J. Friedrichs, M. Mydlack, T. Baumann, M. Nieger and S. Bräse, Chem. Commun., 2013, 49, 6501 RSC.
- (a) D. R. McMillin, M. T. Buckner and B. T. Ahn, Inorg. Chem., 1977, 16, 943 CrossRef; (b) M. T. Buckner and D. R. McMillin, J. Chem. Soc., Chem. Commun., 1978, 759 RSC; (c) M. W. Blaskie and D. R. McMillin, Inorg. Chem., 1980, 19, 3519 CrossRef CAS; (d) D. R. McMillin, J. R. Kirchhoff and K. V. Goodwin, Coord. Chem. Rev., 1985, 64, 83 CrossRef CAS; (e) D. R. Crane, J. DiBenedetto, C. E. A. Palmer, D. R. McMillin and P. C. Ford, Inorg. Chem., 1988, 27, 3698 CrossRef CAS; (f) D. R. McMillin and K. M. McNett, Chem. Rev., 1998, 98, 1201 CrossRef PubMed; (g) A. Lavie-Cambot, M. Cantuel, Y. Leydet, G. Jonusauskas, D. M. Bassani and N. D. McClenaghan, Coord. Chem. Rev., 2008, 252, 2572 CrossRef CAS.
- N. Armaroli, G. Accorsi, F. Cardinali and A. Listorti, Top. Curr. Chem., 2007, 280, 69 CrossRef CAS.
- (a) L. X. Chen, G. Jennings, T. Liu, D. J. Gosztola, J. P. Hessler, D. V. Scaltrito and G. J. Meyer, J. Am. Chem. Soc., 2002, 124, 10861 CrossRef CAS PubMed; (b) L. X. Chen, G. B. Shaw, I. Novozhilova, T. Liu, G. Jennings, K. Attenkofer, G. J. Meyer and P. Coppens, J. Am. Chem. Soc., 2003, 125, 7022 CrossRef CAS PubMed; (c) R. M. Everly and D. R. McMillin, Photochem. Photobiol., 1989, 50, 711 CrossRef CAS; (d) E. M. Stacy and D. R. McMillin, Inorg. Chem., 1990, 29, 393 CrossRef CAS; (e) I. I. Vorontsov, T. Graber, A. Y. Kovalevsky, I. V. Novozhilova, M. Gembicky, Y.-S. Chen and P. Coppens, J. Am. Chem. Soc., 2009, 131, 6566 CrossRef CAS PubMed; (f) A. K. I. Gushurst, D. R. McMillin, C. O. Dietrich-Buchecker and J. P. Sauvage, Inorg. Chem., 1989, 28, 4070 CrossRef CAS; (g) M. T. Miller, P. K. Gantzel and T. B. Karpishin, J. Am. Chem. Soc., 1999, 121, 4292 CrossRef CAS; (h) Q. Zhang, J. Ding, Y. Cheng, L. Wang, Z. Xie, X. Jing and F. Wang, Adv. Funct. Mater., 2007, 17, 2983 CrossRef CAS; (i) D. Chantal, Coord. Chem. Rev., 2015, 282–283, 19 Search PubMed.
- (a) D. V. Scaltrito, D. W. Thompson, J. A. O'Callaghan and G. J. Meyer, Coord. Chem. Rev., 2000, 208, 243 CrossRef CAS; (b) N. Armaroli, Chem. Soc. Rev., 2001, 30, 113 RSC.
- (a) D. G. Cuttell, S. M. Kuang, P. E. Fanwick, D. R. McMillin and R. A. Walton, J. Am. Chem. Soc., 2002, 124, 6 CrossRef CAS PubMed; (b) D. Volz, D. M. Zink, T. Bocksrocker, J. Friedrichs, M. Nieger, T. Baumann, U. Lemmer and S. Bräse, Chem. Mater., 2013, 25, 3414 CrossRef; (c) B. Carlson, G. D. Phelan, W. Kaminsky, L. Dalton, X. Jiang, S. Liu and A. K.-Y. Jen, J. Am. Chem. Soc., 2002, 124, 14162 CrossRef CAS PubMed; (d) T. McCormick, W.-L. Jia and S. Wang, Inorg. Chem., 2006, 45, 147 CrossRef CAS PubMed; (e) R. Czerwieniec, K. Kowalski and H. Yersin, Dalton Trans., 2013, 42, 9826 RSC; (f) H. Ohara, A. Kobayashia and M. Kato, Dalton Trans., 2014, 43, 17317 RSC; (g) D. M. Zink, D. Volz, T. Baumann, M. Mydlak, H. Flügge, J. Friedrichs, M. Nieger and S. Bräse, Chem. Mater., 2013, 25, 4471 CrossRef CAS; (h) L. Qin, Q. Zhang, W. Sun, J. Wang, C. Lu, Y. Cheng and L. Wang, Dalton Trans., 2009, 9388 RSC; (i) J. Min, Q. Zhang, W. Sun, Y. Cheng and L. Wang, Dalton Trans., 2011, 40, 686 RSC; (j) Q. Zang, Q. Zhou, Y. Cheng, L. Wang, D. Ma, X. Jing and F. Wang, Adv. Mater., 2004, 16, 432 CrossRef; (k) Q. Zhang, Q. Zhou, Y. Cheng, L. Wang, D. Ma, X. Jing and F. Wang, Adv. Funct. Mater., 2006, 16, 1203 CrossRef CAS; (l) N. Armaroli, G. Accorsi, M. Holler, O. Moudam, J.-F. Nierengarten, Z. Zhou, R. T. Wegh and R. Welter, Adv. Mater., 2006, 18, 1313 CrossRef CAS; (m) G. Che, Z. Su, W. Li, B. Chu and M. Li, Appl. Phys. Lett., 2006, 89, 103511 CrossRef; (n) J.-L. Chen, X.-F. Cao, W. Gu, H.-R. Wen, L.-X. Shi, G. Rong and P. Luo, Inorg. Chem. Commun., 2011, 14, 1894 CrossRef CAS.
- C. A. Tolman, Chem. Rev., 1977, 77, 313 CrossRef CAS.
- (a) O. Maury and H. le Bozec, Metal-based quadratic nonlinear optical materials, John Wiley & Sons, UK, 2010, vol. 1, pp. 32–35 Search PubMed; (b) O. Maury and H. le Bozec, Acc. Chem. Res., 2005, 38, 691 CrossRef CAS PubMed; (c) C. E. Housecroft and E. C. Constable, Chem. Soc. Rev., 2015, 44, 8386 RSC; (d) M. Freitag, Q. Daniel, M. Pazoki, K. Sveinbjörnsson, J. Zhang, L. Sun, A. Hagfeldt and G. Boschloo, Energy Environ. Sci., 2015, 8, 2634 RSC.
- P. C. Ford, E. Cariati and J. Bourassa, Chem. Rev., 1999, 99, 3625 CrossRef CAS PubMed.
- D. J. Eisler, C. W. Kirby and R. J. Puddephatt, Inorg. Chem., 2003, 42, 7626 CrossRef CAS PubMed.
- (a) R. Czerwieniec, J. Yu and H. Yersin, Inorg. Chem., 2011, 50, 8293 CrossRef CAS PubMed; (b) U. Monkowius, S. Ritter, B. König, M. Zabel and H. Yersin, Eur. J. Inorg. Chem., 2007, 4597 CrossRef CAS; (c) S.-H. Kuang, D. G. Cuttell, D. R. McMillin, P. E. Fanwick and R. A. Walton, Inorg. Chem., 2002, 41, 3313 CrossRef CAS PubMed.
- R. Caris, B. C. Peoples, M. Valderrama, G. Wu and R. Rojas, J. Organomet. Chem., 2009, 694, 1795 CrossRef CAS.
- M. Kranenburg, Y. E. M. van der Burgt, P. C. J. Kamer and P. W. N. M. van Leeuwen, Organometallics, 1995, 14, 3081 CrossRef CAS.
- M. A. Escobar, D. H. Jara, R. A. Tapia, L. Lemus, R. Fröhlich, J. Guerrero and R. S. Rojas, Polyhedron, 2013, 62, 66 CrossRef CAS.
- G. Accorsi, A. Listorti, K. Yoosaf and N. Armaroli, Chem. Soc. Rev., 2009, 38, 1690 RSC.
- G. Scalmani and M. J. Frisch, J. Chem. Phys., 2010, 132, 114110 CrossRef PubMed.
- C. Femoni, S. Muzzioli, A. Palazzi, S. Stagni, S. Zacchini, F. Monti, G. Accorsi, M. Bolognesi, N. Armaroli, M. Massi, G. Valenti and M. Marcaccio, Dalton Trans., 2013, 42, 997 RSC.
- J. Bard and L. R. Faulkner, Electrochemical methods: fundamentals and applications, Wiley, New York, 2nd edn, 2001 Search PubMed.
- (a) L. He, L. Duan, J. Qiao, R. Wang, P. Wei, L. Wang and Y. Qiu, Adv. Funct. Mater., 2008, 18, 2123 CrossRef CAS; (b) R. D. Costa, E. Orti, H. J. Bolink, F. Monti, G. Accorsi and N. Armaroli, Angew. Chem., Int. Ed., 2012, 51, 8178 CrossRef CAS PubMed.
- F. Lafolet, S. Welter, Z. Popovic and L. de Cola, J. Mater. Chem., 2005, 15, 2820 RSC.
- M. W. Mara, K. A. Fransted and L. X. Chen, Coord. Chem. Rev., 2015, 282–283, 2 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- P. J. Hayand and W. R. Wadt, J. Chem. Phys., 1985, 82, 299 CrossRef.
- M. Cossi, V. Barone, B. Mennucci and J. Tomasi, Chem. Phys. Lett., 1998, 286, 253 CrossRef CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Including spectroscopic and crystallographic details (CIF). CCDC 1422058 for 1 and 1422059 for 2. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra20450j |
| ‡ Laser flash photolysis experiments at different excitation power clearly rule out triplet–triplet annihilation processes being responsible for the faster decaying component. |
| This journal is © The Royal Society of Chemistry 2016 |