Grass-like Co2P superstructures: direct synthesis between elements, forming mechanism and catalytic properties†
Abstract
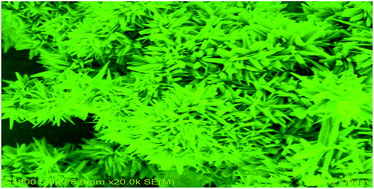
In this paper, a two-step solution process was designed for the preparation of grass-like Co2P superstructures with enhanced catalytic activity. Firstly, cobalt microspheres were solvothermally fabricated through the reaction between N2H4·H2O and CoSO4·7H2O in glycol at 100 °C for 10 h. Then, freshly-prepared Co microspheres reacted with white phosphorus via a hydrothermal route at 180 °C for 16 h to produce grass-like Co2P superstructures. The phase and the morphology of the as-prepared products were characterized by powder X-ray diffraction (XRD), energy dispersive spectrometry (EDS), inductively coupled plasma atomic emission spectroscopy (ICP-AES), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Experiments showed that the as-prepared grass-like Co2P superstructures presented outstanding catalytic activity in the reduction of some aromatic nitro compounds such as 4-nitrophenol, 2-nitrophenol and 4-nitroaniline in excess NaBH4 solution. Simultaneously, several organic dyes including Rhodamine B, Pyronine B and Safranine T could be also rapidly reduced in excess NaBH4 solution, which provides a new choice for the room-temperature decoloration of the dyes.

 Please wait while we load your content...
Please wait while we load your content...