Self-healing, malleable and creep limiting materials using both supramolecular and reversible covalent linkages†
Borui
Zhang
a,
Zachary A.
Digby
a,
Jacob A.
Flum
a,
Elizabeth M.
Foster
a,
Jessica L.
Sparks
b and
Dominik
Konkolewicz
*a
aDepartment of Chemistry and Biochemistry, Miami University, 651 E. High St, Oxford, OH 45056, USA. E-mail: d.konkolewicz@miamiOH.edu
bDepartment of Chemical, Paper, and Biomedical Engineering, Miami University, 650 E. High St, Oxford, OH 45056, USA
First published on 2nd September 2015
Abstract
A self-healing material containing two reversible cross-linkers was made. Relatively rapidly exchanging hydrogen-bonded and slowly exchanging Diels–Alder based cross-linkers were incorporated. Two time scales allowed partial healing at room temperature, and near complete healing upon heating. Slow linkers limited creep at room temperature but allowed reshaping upon heating.
Self-healing materials can intrinsically repair damages and fractures.1–5 Self-healing polymeric materials generally fall into one of two categories: (1) materials that contain capsules or capillaries that lead to chemical reactions upon rupture of the microchamber,6,7 or (2) materials that contain reversible cross-links that can exchange.1,8 Materials that contain dynamic linkages have advantages including simple preparation and the ability to sustain multiple fracture healing cycles.1 Various dynamic linkages have been used for self-healing materials. These include materials based on supramolecular interactions9 such as hydrogen-bond pairs,10,11 host–guest interactions,12 metal coordination,13,14 π-stacking,15,16 and materials based on dynamic covalent cross-links such as Diels–Alder adducts,17–19 disulfide linkages,20,21 radical reshuffling reactions,22–24 boronic esters,25–27etc.
Despite the potential for these materials to be used in various applications, the dynamic nature of the linker introduces the potential for the material to creep or deform over time under load.1 Often, materials that heal quickly through fast exchange of cross-linkers also tend to creep rapidly. This leads to a trade-off between minimizing the healing time and limiting creep. A combination of dynamic and static cross-linkers has been used to overcome this limitation.28–30 In this paper, single-network materials are cross-linked with two dynamic linkers with very different timescales of exchange. Very few examples of doubly dynamic systems have been reported in the literature.31–33 In this contribution, one cross-linker has relatively dynamic hydrogen bonds from the 2-ureido-4[1H]-pyrimidinone (UPy) moiety,34,35 and the other has the significantly less dynamic covalent furan-maleimide (FMI) Diels–Alder adducts.17
UPy linkages have been shown to exchange efficiently in minutes at room temperature,10 while the FMI linkage typically requires heating to >80 °C to have an appreciable rate of exchange.36 By combining both UPy and FMI linkages in the same material, partial healing can be achieved through the UPy linkage at room temperature, with close to full recovery achieved after heating the material. Although hydrogen bonds and Diels–Alder adducts have been recently combined in a single material, the self-healing character of this material was not evaluated extensively.33 The strong covalent bonds in FMI limit the extent of creep, due to slow exchange of these covalent bonds at room temperature.
Self-healing network materials were synthesized using conventional free radical polymerization. The UPy and FMI linkages were introduced into the network through acrylate functionalized UPy (UPyA) and diacrylate functionalized FMI (FMIDA). These monomers were synthesized using procedures adapted from the literature.37–39Scheme 1 describes the proposed healing process. The bulk of the materials (>90% by weight) is poly(2-hydroxyethyl acrylate) (PHEA). PHEA was chosen due to its low glass transition temperature of approx. −15 °C (Tg),40 and the relatively high boiling point of its monomer. The relatively high boiling point of the HEA monomer was important to avoid monomer loss during thermally initiated polymerization that would occur with low boiling monomers.
PHEA based self-healing materials containing both FMIDA and UPyA linkages (PHEA–FMIDA–UPyA) were synthesized with 5 wt% UPyA to HEA, and 2.1 wt% to HEA. This maintains the same average distance between FMIDA and UPyA linkers and gives a molar ratio of [HEA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [UPyA]
[UPyA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [FMIDA] = 130
[FMIDA] = 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Azobis(isobutyronitrile) (AIBN) was used as the radical initiator for the polymerization at 65 °C. The monomer conversion was found to be greater than 90% by gravimetry. Fig. S1a† gives an infrared (IR) spectrum of the material after it has been prepared and dried in a vacuum oven. The IR spectrum shows no peak at 1640 cm−1 (Fig. S1b†), as well as no peak at 940 cm−1. The peaks at 1640 cm−1 and 940 cm−1 are characteristic vibrations of HEA vinyl groups.40 Similarly, Fig. S1c† shows no peak around 1500 cm−1, which corresponds to the C–N stretch in DMF. Other characteristic peaks of DMF including the peaks at 660, 870, 1100 cm−1,41 are also absent from the IR spectrum in Fig. S1(a).† Other peaks of DMF overlap with peaks in HEA such as the C
1. Azobis(isobutyronitrile) (AIBN) was used as the radical initiator for the polymerization at 65 °C. The monomer conversion was found to be greater than 90% by gravimetry. Fig. S1a† gives an infrared (IR) spectrum of the material after it has been prepared and dried in a vacuum oven. The IR spectrum shows no peak at 1640 cm−1 (Fig. S1b†), as well as no peak at 940 cm−1. The peaks at 1640 cm−1 and 940 cm−1 are characteristic vibrations of HEA vinyl groups.40 Similarly, Fig. S1c† shows no peak around 1500 cm−1, which corresponds to the C–N stretch in DMF. Other characteristic peaks of DMF including the peaks at 660, 870, 1100 cm−1,41 are also absent from the IR spectrum in Fig. S1(a).† Other peaks of DMF overlap with peaks in HEA such as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at ∼1700 cm−1, etc.40,41 It is worth noting that the dried PHEA–FMIDA–UPyA material is easily handled, however, it is sticky during the drying process. Further, differential scanning calorimetry (DSC) was used to determine the Tg of the PHEA–FMIDA–UPyA cross-linked polymer. As shown in Fig. S2a† glass transition temperature of approximately 4 °C was measured. This is approximately 20 °C higher than the uncrosslinked material.40 This difference is due to the presence of both UPyA and FMIDA cross-linkers.
O stretch at ∼1700 cm−1, etc.40,41 It is worth noting that the dried PHEA–FMIDA–UPyA material is easily handled, however, it is sticky during the drying process. Further, differential scanning calorimetry (DSC) was used to determine the Tg of the PHEA–FMIDA–UPyA cross-linked polymer. As shown in Fig. S2a† glass transition temperature of approximately 4 °C was measured. This is approximately 20 °C higher than the uncrosslinked material.40 This difference is due to the presence of both UPyA and FMIDA cross-linkers.
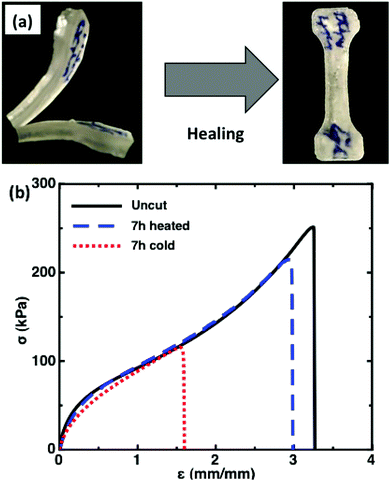
To investigate the potential of the PHEA–FMIDA–UPyA cross-linked polymers to act as self-healing materials, a sample was cut into two pieces, as displayed in the left panel of Fig. 1a. These two pieces were subsequently placed in contact, and the two pieces were joined together by pushing the two halves with fingers for approximately 5 seconds. As shown in right panel of Fig. 1a, the cut material has self-healing character due to the almost complete disappearance of the scratch after 7 h at room temperature.
Tensile-testing was performed to assess the potential of these hybrid UPy/FMI containing networks to act as self-healing materials. Fig. 1b compares the stress–strain curve for a typical uncut material, a material that was cut and allowed to heal at room temperature for 7 h, and a material that was cut and allowed to heal at 90 °C for 7 h. Fig. 1b indicates that the material healed at room temperature for 7 h regained approximately 50% of the strain at break and 50% of the peak stress. Fig. 1b also shows that the material that was heated at 90 °C for 7 h regained ca. 90% of the strain at break and 85% of the peak stress.
These results can be explained as follows: at room temperature (22 °C), the hydrogen bonds in the UPy units, as well as any hydrogen bonds between HEA, are able to exchange. This allows healing up to approximately 50% of the stress and strain of the uncut material. However, at room temperature the Diels–Alder equilibrium is shifted very far to the adduct form implying very little exchange between the covalent units. In contrast, at 90 °C a non-trivial fraction of the Diels–Alder adducts are disassembled, as well as the dynamic hydrogen bonds, allowing exchange in both the UPy and FMI networks. This exchange in both linkers allows the material to recover the majority of its initial mechanical properties. Fig. S3† shows the stress–strain curves for 5 uncut materials, including materials that were heated for 7 h and materials that were not heated. This quantifies the variability in the uncut material. The PHEA–FMIDA–UPyA materials have a relatively low modulus of ∼100 kPa, which is attributed to the relatively low crosslink density.
Fig. S4† shows a high recovery and a low recovery sample that was healed for 7 h at room temperature, and Fig. S5† shows a high recovery and a low recovery sample that was healed for 7 h at 90 °C. These comparisons give a picture of the sample and healing variability which is typically in the range of ∼20–30%. The variability may be attributed to the random scission of bonds upon cutting the sample, with some sections requiring more network rearrangement, and longer healing times, than others. However, for the remainder of this communication the best performing materials will be considered to highlight the potential of these networks.
Interestingly, the uncut and heated materials show an initial regime of negative curvature in the stress strain curve, extending approximately to a strain of ∼1.5. This is interpreted as a region of polymer chain stretching and breaking of the hydrogen-bonded network. Beyond a strain of 1.5 a regime of positive curvature is seen. Interestingly, the material that was healed at room temperature breaks close to the inflection point or transition between these two regimes. This is consistent with the idea that at room temperature only the hydrogen-bonds exchange. In contrast, by heating the material, both hydrogen-bonds and Diels–Alder units are able to exchange, allowing a greater stress and strain at break.
To further analyze the potential of these PHEA–FMIDA–UPyA materials, two forms of damage were applied to the material. One was the complete cut as shown in Fig. 1a and the other was a notch through 50% of the material's thickness. Fig. S6† indicates the nature of the damage does not change the performance as a self-healing material.
Fig. S7a† shows stress–strain curves for materials that have been healed for different times at room temperature, while Fig. S7b† show the kinetics of healing under cold conditions (room temperature). Similarly, Fig. S8a† shows stress–strain curves for materials that have been healed for different times under heated (90 °C) conditions, and Fig. S8b† shows the kinetics of healing under heated conditions (90 °C). These were fitted to an exponential function of the following form for the stress and strain respectively:
| σt = σ∞ − bσexp[−t/τ0] | (1) |
| εt = ε∞ − bεexp[−t/τ0] | (2) |
These data are consistent with the relatively rapid but incomplete healing at room temperature, caused by exchange of the hydrogen-bonded UPy units.10 The healing half-life is expected to be a combination of both the timescale of exchange between UPy units and the timescale of exchange between clusters that have been reported in supramolecular self-healing systems.9 Similarly, the high temperature system is consistent with almost complete healing, since the σ∞ and ε∞ values are similar to the maximum stress and strain at break for the uncut material (Fig. S3†). However, this healing occurs at a much slower rate with a half-life of about 2.2 h at 90 °C.
The PHEA–FMIDA–UPyA material performs well as a self-healing material; however, an important consideration is the tendency to deform and creep over time under load. Fig. 2a shows both the stress relaxation at constant extension (100% strain) and the creep deformation at constant stress (100 kPa) over a 4 h time period for the PHEA–FMIDA–UPyA system. Although stress relaxation and creep occurs initially, both plateau at approximately 2.5 h. After this time the material displays negligible stress relaxation or creep. This can be explained by the stress relaxation and creep occurring primarily in the hydrogen-bonded network, which exchanges on the timescale of minutes at room temperature. The Diels–Alder network ensures that the material maintains its structure at room temperature and limits creep and stress relaxation.
It is important to note that the creep and stress-relaxation experiments on the PHEA–FMIDA–UPyA system showed that there is some extent of exchange under extension/stress. To determine whether there is permanent deformation over a longer period of time, the PHEA–FMIDA–UPyA material was stretched to 100% extension, and fixed at 100% extension for 7 days. After this time, the material was released from the 100% extension and allowed to relax. The length of the material (L) after being released was compared to the original length before extension (L0) at several time points. Fig. 2b shows the kinetics of L/L0 after the fixed strain was released. The kinetic data indicates that the recovery was better than 95% after 2 hours, and recovery was complete after 16 h. Similar experiments were performed where the sample was kept at 100% extension for 1, 3 and 7 days. As shown in the inset to Fig. 2b, 16 h after the strain was released, the materials fixed in place for 1, 3 and 7 days all returned to their original lengths.
Finally, the dynamic nature of double-self healing material was exploited to reshape the material, displaying its malleable properties. This is similar to the “vitrimer” materials, which operate through a dynamic exchange rather than an association/dissociation dynamic mechanism.42–44 In particular, by incubating the material at elevated temperature both the hydrogen-bonded cross-links and the Diels–Alder cross-links exchange at a non-trivial rate. The material can be reshaped by twisting the material at room temperature and fixing it in this configuration while heating it at 90 °C. Fig. 2c shows the shape of the material both directly after synthesis (left), after being heated for 7 h at 90 °C in a twisted configuration (middle).
Clearly, the original untwisted shape is changed to a heavily twisted shape after heating in the twisted configuration. Finally, since the material is >90% PHEA, a material that forms hydrogels,45 the reshaped material was placed in to flask of water for 1 h at room temperature to assist with displaying the permanent structure. As shown in Fig. 2c (right) the material retains its new twisted shape, even after being placed in the water bath for an hour.
Conclusion
In conclusion, a new class of self-healing materials has been synthesized to contain both highly dynamic but relatively weakly bound supramolecular cross-links and strong slowly exchanging covalent cross-links. In this system, hydrogen bonds were introduced to create the highly dynamic cross-links, and covalent Diels–Alder linkages were introduced to create the slowly exchanging linkages. This material shows partial healing at room temperature and shows almost complete recovery at elevated temperatures. An important feature of these materials is that the very slowly exchanging Diels–Alder cross-links lead to a limited extent of creep, and complete recovery once the stress is released. However, due to the dynamic nature of the material, it is malleable at elevated temperatures and its permanent shape can be changed.Acknowledgements
We are grateful to Jason Berberich for fruitful discussions. D. K. acknowledges Start Up funding. D. K. and B. Z. acknowledge CFR funding from Miami University. Z. D. acknowledges support from a USS scholarship.References
- N. Roy, B. Bruchmann and J.-M. Lehn, Chem. Soc. Rev., 2015, 44, 3786 RSC.
- D. Y. Wu, S. Meure and D. Solomon, Prog. Polym. Sci., 2008, 33, 479 CrossRef CAS PubMed.
- J. A. Syrett, C. R. Becer and D. M. Haddleton, Polym. Chem., 2010, 1, 978 RSC.
- Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446 RSC.
- W. H. Binder, Self-healing polymers: from principles to applications, John Wiley & Sons, 2013 Search PubMed.
- S. R. White, N. R. Sottos, P. H. Geubelle, J. S. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown and S. Viswanathan, Nature, 2001, 409, 794 CrossRef CAS PubMed.
- K. S. Toohey, N. R. Sottos, J. A. Lewis, J. S. Moore and S. R. White, Nat. Mater., 2007, 6, 581 CrossRef CAS PubMed.
- R. J. Wojtecki, M. A. Meador and S. J. Rowan, Nat. Mater., 2011, 10, 14 CrossRef CAS PubMed.
- F. Herbst, D. Döhler, P. Michael and W. H. Binder, Macromol. Rapid Commun., 2013, 34, 203 CrossRef CAS PubMed.
- J. Cui and A. d. Campo, Chem. Commun., 2012, 48, 9302 RSC.
- M. Guo, L. M. Pitet, H. M. Wyss, M. Vos, P. Y. W. Dankers and E. W. Meijer, J. Am. Chem. Soc., 2014, 136, 6969 CrossRef CAS PubMed.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef PubMed.
- M. Burnworth, L. Tang, J. R. Kumpfer, A. J. Duncan, F. L. Beyer, G. L. Fiore, S. J. Rowan and C. Weder, Nature, 2011, 472, 334 CrossRef CAS PubMed.
- G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176 CrossRef CAS PubMed.
- S. Burattini, B. W. Greenland, D. H. Merino, W. Weng, J. Seppala, H. M. Colquhoun, W. Hayes, M. E. Mackay, I. W. Hamley and S. J. Rowan, J. Am. Chem. Soc., 2010, 132, 12051 CrossRef CAS PubMed.
- S. Burattini, H. M. Colquhoun, B. W. Greenland and W. Hayes, Faraday Discuss., 2009, 143, 251 RSC.
- X. Chen, M. A. Dam, K. Ono, A. Mal, H. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295, 1698 CrossRef CAS PubMed.
- P. Reutenauer, E. Buhler, P. J. Boul, S. J. Candau and J. M. Lehn, Chem. – Eur. J., 2009, 15, 1893 CrossRef CAS PubMed.
- D. N. Amato, G. A. Strange, J. P. Swanson, A. D. Chavez, S. E. Roy, K. L. Varney, C. A. Machado, D. V. Amato and P. J. Costanzo, Polym. Chem., 2014, 5, 69 RSC.
- J. Canadell, H. Goossens and B. Klumperman, Macromolecules, 2011, 44, 2536 CrossRef CAS.
- J. A. Yoon, J. Kamada, K. Koynov, J. Mohin, R. Nicolaÿ, Y. Zhang, A. C. Balazs, T. Kowalewski and K. Matyjaszewski, Macromolecules, 2011, 45, 142 CrossRef.
- Y. Amamoto, J. Kamada, H. Otsuka, A. Takahara and K. Matyjaszewski, Angew. Chem., Int. Ed., 2011, 50, 1660 CrossRef CAS PubMed.
- Y. Amamoto, H. Otsuka, A. Takahara and K. Matyjaszewski, Adv. Mater., 2012, 24, 3975 CrossRef CAS PubMed.
- H. Y. Park, C. J. Kloxin, T. F. Scott and C. N. Bowman, Macromolecules, 2010, 43, 10188 CrossRef CAS PubMed.
- J. J. Cash, T. Kubo, A. P. Bapat and B. S. Sumerlin, Macromolecules, 2015, 48, 2098 CrossRef CAS.
- C. C. Deng, W. L. A. Brooks, K. A. Abboud and B. S. Sumerlin, ACS Macro Lett., 2015, 4, 220 CrossRef CAS.
- O. R. Cromwell, J. Chung and Z. Guan, J. Am. Chem. Soc., 2015, 137, 6492 CrossRef CAS PubMed.
- B. V. S. Iyer, I. G. Salib, V. V. Yashin, T. Kowalewski, K. Matyjaszewski and A. C. Balazs, Soft Matter, 2013, 9, 109 RSC.
- F. Sordo, S.-J. Mougnier, N. Loureiro, F. Tournilhac and V. Michaud, Macromolecules, 2015, 48, 4394–4402 CrossRef CAS.
- D. Döhler, H. Peterlik and W. H. Binder, Polymer, 2015, 69, 264 CrossRef PubMed.
- N. Roy, E. Buhler and J.-M. Lehn, Polym. Int., 2014, 63, 1400 CrossRef CAS PubMed.
- J. A. Neal, D. Mozhdehi and Z. Guan, J. Am. Chem. Soc., 2015, 137, 4846 CrossRef CAS PubMed.
- R. Araya-Hermosilla, A. A. Broekhuis and F. Picchioni, Eur. Polym. J., 2014, 50, 127 CrossRef CAS PubMed.
- B. J. B. Folmer, R. P. Sijbesma, R. M. Versteegen, J. A. J. van der Rijt and E. W. Meijer, Adv. Mater., 2000, 12, 874 CrossRef CAS.
- K. E. Feldman, M. J. Kade, E. W. Meijer, C. J. Hawker and E. J. Kramer, Macromolecules, 2009, 42, 9072 CrossRef CAS.
- S. D. Bergman and F. Wudl, J. Mater. Chem., 2008, 18, 41 RSC.
- H. M. Keizer, R. van Kessel, R. P. Sijbesma and E. W. Meijer, Polymer, 2003, 44, 5505 CrossRef CAS.
- H. M. Janssen, G. M. L. V. Gemert, A. T. T. Cate, D. J. M. V. Beek, R. P. Sijbesma, E. W. Meijer and A. W. Bosman, US Pat., 20040034190A1, 2004 Search PubMed.
- J. A. Syrett, G. Mantovani, W. R. S. Barton, D. Price and D. M. Haddleton, Polym. Chem., 2010, 1, 102 RSC.
- E. Vargün and A. Usanmaz, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3957 CrossRef PubMed.
- A. Sharma, S. Kaur, C. G. Mahajan, S. K. Tripathi and G. S. S. Saini, Mol. Phys., 2007, 105, 117 CrossRef CAS PubMed.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965 CrossRef CAS PubMed.
- M. Capelot, D. Montarnal, F. Tournilhac and L. Leibler, J. Am. Chem. Soc., 2012, 134, 7664 CrossRef CAS PubMed.
- J. P. Brutman, P. A. Delgado and M. A. Hillmyer, ACS Macro Lett., 2014, 3, 607 CrossRef CAS.
- M. Monleón Pradas, J. L. Gómez Ribelles, A. Serrano Aroca, G. Gallego Ferrer, J. Suay Antón and P. Pissis, Polymer, 2001, 42, 4667 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, additional characterization data. See DOI: 10.1039/c5py01214g |
| This journal is © The Royal Society of Chemistry 2015 |