Task-specific ionic liquids for the extraction of metal ions from aqueous solutions
Ann E. Vissera, Richard P. Swatloskia, W. Matthew Reicherta, Rebecca Maytonc, Sean Sheffc, Andrzej Wierzbickic, James H. Davis, Jr.*a and Robin D. Rogers*a
aCenter for Green Manufacturing, The University of Alabama, Tuscaloosa, AL 35487, USA.. E-mail: E-mail: RDRogers@bama.ua.edu
bDepartment of Chemistry, The University of Alabama, Tuscaloosa, AL 35487, USA
cDepartment of Chemistry, University of South Alabama, Mobile, AL 36688, USA.. E-mail: JDavis@jaguar1.usouthal.edu
First published on UnassignedUnassigned3rd January 2001
Abstract
Imidazolium cations, such as those commonly used in preparing ionic liquids (ILs) can easily be derivatized to include task-specific functionality, such as metal ligating groups that when used as part of the solvent or doped into less expensive ILs, dramatically enhance the partitioning of targeted metal ions into the IL phase from water; the strategy of preparing task-specific ILs is applicable to a wide range of designer solvent needs.
Owing to their unique chemical and physical properties, ionic liquids (ILs) have received recent attention for applications as solvent alternatives,1–4 where, for example, ILs can be used in place of organic solvents in synthesis, catalysis, electrochemistry and liquid/liquid extractions. The formulations commonly reported for ILs have relied on pyridinium or imidazolium cations bearing simple alkyl appendages as the cation. Changes in IL physical properties have been accomplished by altering the length of the alkyl groups on the rings4–6 allowing for fine-tuning their viscosity, hydrophobicity and melting points.6,7 More recently, ionic liquid formulations have been expanded in scope to include other heterocyclic aromatic molecules as well as ions with structurally and functionally complex side chains.8–10
It has been demonstrated that organic solutes (e.g. aromatic molecules such as simple benzene derivatives)1,2,4 can be partitioned to specific ILs based on the hydrophobicity of the solute and IL. In contrast, the partitioning of metal ions into an IL extracting phase in liquid/liquid systems is negligible owing to the tendency of the metal cations to remain hydrated and in the aqueous phase, thus necessitating the use of an extractant molecule that forms complexes directly with metal ions to increase their hydrophobicity.11–15 The drawbacks associated with this approach lie in finding extractant molecules that remain exclusively in the IL under all process conditions and also in understanding the increased complexity of the system upon the addition of solutes. Here, we report the first use of task-specific ILs, i.e. those with targeted functionality designed into the IL solvent. In the present example, new compounds which have been designed specifically to both be ionic liquids and to extract heavy metal ions (e.g. Hg2+ and Cd2+) are reported.
Mercury(II) and cadmium(II) were targeted in this study as part of our ongoing efforts to find alternative separations strategies for removing these toxic, easily transported metal ions from the environment.16 The bases for the modified ILs were 1-alkyl-3-methylimidazolium, Cnmim+ (n = 4, 6, 8) salts of PF6− which form two-phase systems when contacted in equal volume with water.2 ILs that incorporated thiourea, thioether and urea into derivatized imidazolium cations were thus prepared,† that when combined with the PF6− anion, functioned as both the hydrophobic solvent and metal ion extractant in liquid/liquid separations. The new ILs (Fig. 1) may either be used directly as the bulk solvent or may be doped‡ as an extractant into less expensive ILs, such as [C4mim][PF6].
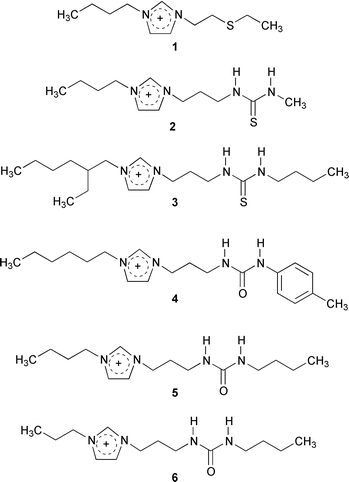 | ||
| Fig. 1 Structures of the cations combined with PF6− to make the ILs utilized in these studies. | ||
The distribution ratios§ of Hg2+ and Cd2+ between our chosen standard for this study, [C4mim][PF6], and an aqueous phase at pH = 7 were 0.84 (Hg2+) and 0.03 (Cd2+), indicating a preference for these metal ions to be retained in the aqueous phase. The thioether-appended IL 1, as either the extracting phase or in 50∶50 mixtures with [C4mim][PF6] at pH = 1 or 7, is effective in partitioning both metal ions to the IL phase (Table 1). The thiourea derivative 2 extracts Hg2+ from water comparably to 1, however distribution ratios are much lower when 2 is diluted with [C4mim][PF6]. IL 2 is much less effective at extraction of Cd2+ (D values of 20 and 23 at pH of 1 and 7, respectively) and when diluted with [C4mim][PF6], IL 2 does not extract Cd2+ at all.
| IL | M2+ | pH (aq) | Distribution ratio | System |
|---|---|---|---|---|
| 1 | Hg2+ | 1 | 200 | 1 only |
| Cd2+ | 1 | 330 | 1 only | |
| 1 | Hg2+ | 1 | 170 | 1 + [C4mim][PF6] (1∶1) |
| Cd2+ | 1 | 310 | 1 + [C4mim][PF6] (1∶1) | |
| 1 | Hg2+ | 7 | 210 | 1 only |
| Cd2+ | 7 | 380 | 1 only | |
| 1 | Hg2+ | 7 | 210 | 1 + [C4mim][PF6] (1∶1) |
| Cd2+ | 7 | 360 | 1 + [C4mim][PF6] (1∶1) | |
| 2 | Hg2+ | 1 | 350 | 2 only |
| Cd2+ | 1 | 20 | 2 only | |
| 2 | Hg2+ | 1 | 74 | 2 + [C4mim][PF6] (1∶1) |
| Cd2+ | 1 | 0.0086 | 2 + [C4mim][PF6] (1∶1) | |
| 2 | Hg2+ | 7 | 340 | 2 only |
| Cd2+ | 7 | 23 | 2 only | |
| 2 | Hg2+ | 7 | 100 | 2 + [C4mim][PF6] (1∶1) |
| Cd2+ | 7 | 0.0074 | 2 + [C4mim][PF6] (1∶1) |
IL 3 (another thiourea derivative), and 4–6 (urea derivatives) were prepared and their behavior as extractants for Hg2+ and Cd2+ when mixed in equal mass ratios with [C4mim][PF6] measured as a function of aqueous phase pH (Fig. 2). The results are similar, in that each of these ILs {as 1∶1 (mass) mixtures with [C4mim][PF6]} efficiently extract Cd2+ and Hg2+, as shown in Fig. 2. In general, the distribution ratios of Cd are lower than observed for Hg, and in the case of ILs 4 and 6, the differences are over an order of magnitude. ILs 3 and 5 give the highest distribution ratios for each metal ion, and the extraction using IL 6 is the most affected by lowering the pH (although all four ILs exhibit lower distribution rations at the lowest values of pH studied). In comparing these results with those in Table 1, it is to be noted that distribution ratios as high as 710 (Hg2+, IL 3) were observed in this latter study, even though the ILs were utilized as 1∶1 mixtures with [C4mim][PF6].
![Distribution ratios for Hg2+ (filled symbols) and
Cd2+ (open symbols) with ILs 3–6
utilized in a 1∶1 weight ratio with
[C4mim][PF6] and contacted with aqueous phases of
variable pH.](/image/article/2001/CC/b008041l/b008041l-f2.gif) | ||
| Fig. 2 Distribution ratios for Hg2+ (filled symbols) and Cd2+ (open symbols) with ILs 3–6 utilized in a 1∶1 weight ratio with [C4mim][PF6] and contacted with aqueous phases of variable pH. | ||
Both the appended functional group and the alkyl group appear to affect the extraction. The extended alkyl ‘tail’ near the thiourea group in 3 results in a significant increase in D values for both metals. For the various functional groups, the D values for Hg2+ are the highest with urea > thiourea with the ‘tail’ > thioether > thiourea, while those for Cd2+ decrease from thiourea with the ‘tail’ > thioether > urea > thiourea.
We are currently investigating additional series of task-specific ILs with each functional group to elucidate the trends in both structure and function of the IL and to control the physical properties of these new extracting solvents. Additional studies are also required to determine if the metal ions can be effectively stripped from these solvents, although they are currently also being investigated for their ability to retain metallic catalysts in the IL for synthetic applications.
Acknowledgements
This work was supported by funding from the Division of Chemical Sciences, Office of Basic Energy Sciences, Office of Energy Research, U.S. Department of Energy (R. D. R., Grant No. DE-FG02-96ER14673), the PG Research Foundation (R. D. R), Research Corporation (J. H. D., Grant CC 4758), the Alabama Supercomputer Authority (A. W.), and the Nichols Corporation (A. W.).Notes and references
- L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 1999, 399, 28 CrossRef
.
- J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 1765 RSC
.
- M. Freemantle,
Chem. Eng. News, 1998, 76(March 30) 32. Search PubMed
.
- A. E. Visser, R. P. Swatloski and R. D. Rogers, Green Chem., 2000, 1, 1 RSC
.
- C. M. Gordon, J. D. Holbrey, A. R. Kennedy and K. R. Seddon, J. Mater. Chem., 1998, 8, 2627 RSC
.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133 RSC
.
- P. Bonĥote, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168 CrossRef CAS
.
- J. H. Davis, Jr., K. J. Forrester and T. J. Merrigan, Tetrahedron Lett., 1998, 39, 8955 CrossRef
.
- J. H. Davis, Jr. and K. J. Forrester, Tetrahedron Lett., 1999, 40, 1621 CrossRef CAS
.
- T. K. Merrigan, E. D. Bates, S. C. Dorman and J. H. Davis, Jr., Chem. Commun., 2000, 2051 RSC
.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, S. T. Griffin and R. D. Rogers, Ind. Eng. Chem. Res., 2000, 39, 3596 CrossRef CAS
.
- S. Dai, Y. H. Ju and C. E. Barnes, J. Chem. Soc., Dalton Trans., 1999, 1201 RSC
.
- A. E. Visser,
R. P. Swatloski,
D. H. Hartman,
J. G. Huddleston and
R. D. Rogers, in
Calixarene Molecules for Separations, ed. G. J. Lumetta, R.
D. Rogers and A. S. Gopalan, American Chemical Society,
Washington, DC, ACS Symp. Ser. 757, 2000,
p. 223. Search PubMed
.
- R. D. Rogers,
A. E. Visser,
R. P. Swatloski and
D. H. Hartman, in
Metal Separation Technologies Beyond 2000: Integrating Novel
Chemistry with Processing, ed. K. C. Liddell and D. J. Chaiko,
The Minerals, Metallurgical and Materials Society,
Warrendale, PA, 1999, p.
139. Search PubMed
.
- A. E. Visser,
R. P. Swatloski,
S. T. Griffin,
D. H. Hartman and
R. D. Rogers,
Sep. Sci. Technol., 2000, in
press. Search PubMed
.
- R. D. Rogers and S. T. Griffin, J. Chromatogr. B, 1998, 711, 277 CrossRef CAS
.
Footnotes |
| † All chemicals were obtained
as reagent grade from Aldrich and used without further purification.
1-(3′-aminopropyl)imidazole (20.0 g, 0.160 mol) was mixed with 100 mL
of acetonitrile under an atmosphere of dry nitrogen. To the stirred
solution was added in a dropwise fashion 15.5 g (0.156 mol) of
n-butyl isocyanate dissolved in 25 mL of acetonitrile. The
combined solution was stirred overnight followed by removing the solvent
in vacuo. The residue was then dried overnight in vacuo.
Proton NMR spectroscopy confirmed the structure of the product as being the
desired urea-appended imidazole, and the crude material was used in the
next step without further purification. Under a nitrogen atmosphere, the reaction residue was redissolved in acetonitrile (100 mL) and 28.0 g (0.164 mol) of propyl iodide was added. The mixture was then heated gently without refluxing. After stirring with heating overnight, the acetonitrile was removed in vacuo, leaving a sticky residue. The residue was washed in water and the aqueous layer washed twice with 100 mL of diethyl ether. To the aqueous solution was added a solution of 36.0 g (0.194 mol) KPF6 in 100 mL of water. The mixture was stirred overnight at 40 °C, during which time a biphasic system formed comprised of an upper aqueous phase and a lower product phase. (Anion exchange for each of the ureas can also be accomplished using AgPF6 in acetone.) The aqueous phase was decanted and the product was washed four times (2 h contacts) with 100 mL water to remove any remaining KPF6. After the last water wash, the ionic liquid was dissolved in acetonitrile and toluene was added to aid in the azeotropic removal of water. Any solids were removed by filtration and the solution was then rotary evaporated. The isolated product was dried in vacuo for 24 h while being heated to 60 °C. Unoptimized yield: 45.2 g (68%). The general procedure for the preparation of the urea-functionalized ionic liquids 5 and 6 and the thiourea-functionalized ionic liquids 2 and 3 is analogous, each being formed in similar yield. |
| ‡ When preparing the 1∶1 mixtures of the solid samples with [C4mim][PF6], solutions of 5 and 6 were prepared as a 1∶1 ratio of the cations and added to [C4mim][PF6] followed by sonication and gentle heating for 30 min to form the solution. Solutions of 1–4 were prepared as 1∶1 weight ratios in [C4mim][PF6] followed by thorough mixing. |
| § Metal ion distribution ratios were determined by mixing equal volumes of the IL and aqueous phases followed by vortexing (2 min) and centrifuging (2000 g, 2 min) to equilibrate the phases. Addition of either 203HgCl2 or 109CdCl2 (ca. 0.005 μCi, 5 μL) was followed by two intervals of vortexing (2 min) and centrifuging (2000 g, 2 min) to ensure that the phases were fully separated. The phases were separated and dispensed into shell vials from which 100 μL of each phase was removed for radiometric analysis. The results are reported as distribution ratios and are calculated as the radioactivity in the lower phase divided by the radioactivity in the upper phase. Each experiment was done in duplicate and the results agreed to within 5%. |
| This journal is © The Royal Society of Chemistry 2001 |
