Enantioselective hydrolysis of amino acid esters by apomyoglobin: perfect kinetic resolution of a phenylalanine derivative
Katsuhiko Tomisaka, Yasuhiro Ishida, Katsuaki Konishi and Takuzo Aida*
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656, Japan.. E-mail: aida@macro.t.u-tokyo.ac.jp;; Fax: +81-3-5841-7310
First published on 18th December 2000
Abstract
Apoprotein of horse-heart myoglobin promoted enantioselective hydrolysis of 4-nitrophenyl esters of amino acids, which allowed nearly perfect kinetic resolution of racemic N-Boc-phenylalanine ester (Boc-Phe-ONp).
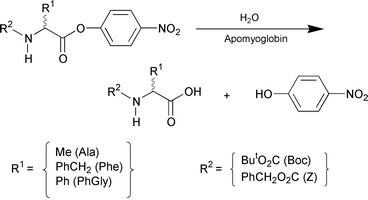
Protein catalysis has a potential for asymmetric synthesis.1 Apoproteins of cofactor-dependent proteins have attracted attention as the new candidates, since they possess a chiral hydrophobic cavity which can bind asymmetric substrates stereoselectively.2,3 However, the successful examples are limited. Recently, we have found that the apoprotein of cytochrome b562 binds chiral porphyrins enantioselectively4 and catalyzes template-assisted metalation of porphyrins.5 Herein we report a novel asymmetric catalysis of apomyoglobin in hydrolysis of amino acid esters,6 and wish to highlight an extremely high enantioselectivity for N-Boc-phenylalanine 4-nitrophenyl ester (Scheme 1).
 | ||
| Scheme 1 | ||
Apomyoglobin is one of the most extensively studied apoproteins,7 and has been reported to catalyze hydrolysis of 4-nitrophenyl alkanoates.8 Horse-heart myoglobin was treated with dilute hydrochloric acid and butanone to give apomyoglobin, which was subjected to extensive dialysis against phosphate buffer (pH = 7.0, 10 mM).9 Hydrolysis of N-Boc-phenylalanine 4-nitrophenyl ester (Boc-Phe-ONp) (Scheme 1) was investigated in a phosphate buffer containing 1% dioxane at 4 °C,10 where the uncatalyzed hydrolysis ([Boc-Phe-ONp]0 = 6.25 μM) proceeded sluggishly (Fig. 1d; △) to furnish only 11% conversion even after 240 min.11 On the other hand, addition of apomyoglobin to the system resulted in a considerable acceleration of the reaction, where the L-isomer was preferentially hydrolyzed over the D-isomer. For example, when racemic Boc-Phe-ONp (12.5 μM) was mixed with apomyoglobin (62.5 μM), the hydrolysis proceeded smoothly to reach 60% conversion in only 8 min. Upon mixing of the reaction mixture with ether or ethyl acetate, the products (Boc-Phe-OH and 4-nitrophenol) and unreacted Boc-Phe-ONp were completely extracted into the organic phase. Chiral HPLC analysis of the organic phase with Chiralcel™ OD-H or Sumichiral™ OA-4700 showed that unreacted Boc-Phe-ONp is predominantly the D-isomer with an L∶D ratio of 1∶99 (98% ee), while the hydrolyzed product (Boc-Phe-OH) has an L∶D ratio of 18∶82. Thus, the hydrolysis with apomyoglobin enables nearly perfect kinetic resolution of racemic Boc-Phe-ONp.
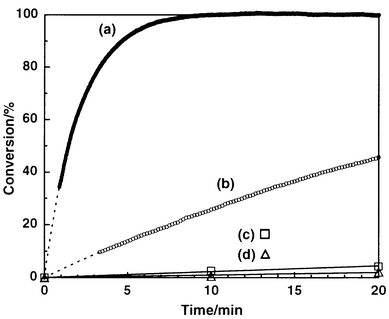 | ||
| Fig. 1 Time courses of the hydrolysis of Boc-Phe-ONp in phosphate buffer (pH 7.0, 10 mM) containing 1% dioxane at 4 °C: (a) Boc-L-Phe-ONp–apomyoglobin (12.5∶62.5 μM), (b) Boc-D-Phe-ONp–apomyoglobin (12.5∶62.5 μM), (c) Boc-Phe-ONp–imidazole (6.25∶62.5 μM) and (d) Boc-Phe-ONp (6.25 μM) without catalyst. | ||
The hydrolysis of Boc-Phe-ONp (62.5 μM) also proceeded to attain 100% conversion by using an equimolar amount of apomyoglobin. Furthermore, upon addition of a fresh feed of the substrate (0.2 equiv.) to this reaction mixture, the hydrolysis ensued without significant loss of the activity, indicating that the reaction turns over catalytically with respect to apomyoglobin. The hydrolysis most likely occurs via acyl transfer to an imidazole group located within the heme pocket. In fact, the reaction was not accelerated by holomyoglobin, whose heme pocket is blocked by the native guest.8 On the other hand, when imidazole was used as a reference catalyst, Boc-Phe-ONp was hydrolyzed much more slowly than with apomyoglobin. For example, the hydrolysis in the presence of 10 equiv. of imidazole (62.5 μM) with respect to Boc-Phe-ONp proceeded to 4% conversion in 20 min (Fig. 1c; □), where the pseudo first-order rate constant (kobs = 1.4 × 10−3 min−1) was only 2.7 times as high as that of the uncatalyzed reaction (kuncat = 5.2 × 10−4 min−1) (Fig. 1d; △).12
In order to obtain further insight into the enantioselective catalysis of apomyoglobin, the kinetics of the hydrolysis of the L- and D-isomers of Boc-Phe-ONp (12.5 μM) were investigated in the presence of apomyoglobin (62.5 μM). When Boc-L-Phe-ONp was the substrate, the hydrolysis took place smoothly to give Boc-L-Phe-OH and 4-nitrophenol quantitatively within only 10 min (Fig. 1a). The pseudo first-order rate constant (kLobs) was 4.9 × 10−1 min−1, which is 940 times larger than that in the absence of the apoprotein. In contrast, hydrolysis of the D-isomer proceeded very slowly (Fig. 1b) with a rate constant (kDobs) of only 3.1 × 10−2 min−1 (kDobs/kuncat = 59), which is 16 times smaller than that of the L-isomer under the same conditions.
Upon increasing the initial concentration of the apoprotein from 25 to 125 μM, the hydrolysis rates of Boc-L-Phe-ONp and Boc-D-Phe-ONp both showed a saturation signature (Fig. 2a), indicating that the apoprotein and Boc-Phe-ONp form a complex which serves as a reactive intermediate. Lineweaver–Burk plots11,13 gave a maximum rate constant for the L-isomer (kLcat) of 6.1 × 10−1 min−1, which is approximately 16 times larger than that for the D-isomer (kDcat = 3.8 × 10−2 min−1) (Fig. 2b).14 On the other hand, the Km value for the hydrolysis of Boc-L-Phe-ONp (KLm) was evaluated to be 1.5 × 10−5 M, which is comparable to that of the D-isomer (KDm = 1.4 × 10−5 M).12 Thus, the high stereoselectivity of the hydrolysis is not due to the enantioselective binding of the substrate (KLm−1/KD m−1 = 0.95) but is more likely due to the large difference in reactivity between the L- and D-isomers of Boc-Phe-ONp within the heme pocket.
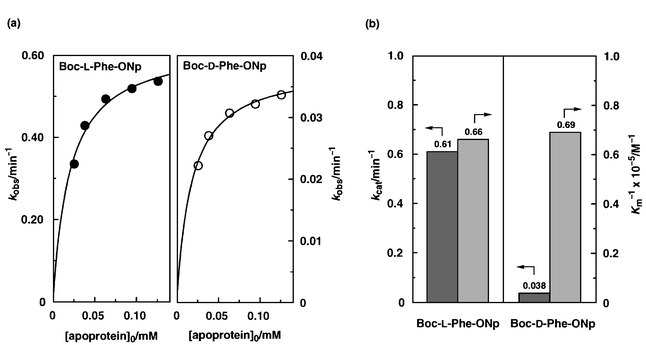 | ||
| Fig. 2 Hydrolysis of Boc-L-Phe-ONp and Boc-D-Phe-ONp (12.5 μM) in the presence of apomyoglobin (2–10 equiv.) in phosphate buffer (pH 7.0, 10 mM) containing 1% dioxane at 4 °C: (a) plots of hydrolysis rate constant (kobs) vs. initial concentration of apomyoglobin, and (b) association constants of the Michaelis complexes (Km−1) and maximum rate constants (kcat). | ||
The enantioselectivity of the apomyoglobin-mediated hydrolysis was found to be highly sensitive to the substrate. For example, when the N-protecting Boc group (R2) of the substrate was replaced with benzyloxycarbonyl (Z-Phe-ONp, 12.5 μM), the rate constant of the hydrolysis of the L-isomer with apomyoglobin (62.5 μM) was considerably decreased (kLobs = 5.5 × 10−2 min−1, kDobs = 3.1 × 10−2 min−1) to furnish a kLobs∶kDobs of only 1.8.15 Lower enantioselectivities were also observed when Boc-PhGly-ONp (R1 = Ph, kLobs/kDobs = 1.8)16 and Boc-Ala-ONp (R1 = Me, kLobs/kDobs = 1.9)16 were the substrates in place of Boc-Phe-ONp, although the L-isomers were again preferentially hydrolyzed.
In conclusion, through the present studies on the utilization of the apoprotein of horse-heart myoglobin as a potential chiral catalyst, we have demonstrated a highly enantioselective hydrolysis of a phenylalanine 4-nitrophenyl ester (Boc-Phe-ONp), whose selectivity is one of the highest reported to date. Exploration with other natural and mutated apoproteins is one of the subjects worthy of further investigation for extending the scope of reactions.
Acknowledgements
We thank JSPS for financial support. Y. I. thanks the JSPS Young Scientist Fellowship.Notes and references
- E. T. Kaiser and D. S. Lawrence, Science, 1984, 226, 505 CrossRef CAS; (a) G. M. Whitesides and C.-H. Wong, Angew. Chem., Int. Ed. Engl., 1985, 24, 617 CrossRef; (b) P. G. Schultz and R. A. Lerner, Acc. Chem. Res., 1993, 26, 391 CrossRef CAS; (c) R. O. Duthaler, Tetrahedron, 1994, 50, 1539 CrossRef CAS.
- (a) U. G. Wagner, N. Müller, W. Schmitzberger, H. Falk and C. Kratky, J. Mol. Biol., 1995, 247, 326 CrossRef CAS; (b) B. B. Lakshmi and C. R. Martin, Nature, 1997, 388, 758 CrossRef CAS.
- For utilization of protein hydrophobic domains for catalysis, see: (a) M. Nango, Y. Kimura, S. Kanda, Y. Ihara, J. Koga and N. Kuroki, Chem. Lett., 1986, 229 CAS; (b) F. Hollfelder, A. J. Kirby and D. S. Tawfik, Nature, 1996, 383, 60 CrossRef CAS; (c) H. Kuang and M. D. Distefano, J. Am. Chem. Soc., 1998, 120, 1072 CrossRef CAS.
- Y. Ishida, K. Konishi, T. Aida and T. Nagamune, Chem. Eur. J., 1998, 4, 1148 CrossRef CAS.
- Y. Ishida, K. Konishi, T. Nagamune and T. Aida, J. Am. Chem. Soc., 1999, 121, 7947 CrossRef CAS.
- Examples of enantioselective hydrolysis of amino acid esters, see: (a) J.-J. Béchet, A. Dupaix and C. Roucous, Biochemistry, 1973, 12, 2566 CrossRef CAS; (b) M. Tanihira and Y. Imanishi, Polym. J., 1983, 15, 499 Search PubMed; (c) T. Miyazawa, H. Iwanaga, S. Ueji, T. Yamada and S. Kuwata, J. Chem. Soc., Chem. Commun., 1988, 1214 RSC; (d) R. Ueoka, Y. Matsumoto, K. Harada, H. Akahoshi, Y. Ihara and Y. Kato, J. Am. Chem. Soc., 1992, 114, 8339 CrossRef CAS; (e) ref 3a..
- (a) Y. V. Griko, P. L. Privalov and S. Y. Venyaminov, J. Mol. Biol., 1988, 202, 127 CrossRef CAS; (b) F. M. Hughson, P. E. Wright and R. L. Baldwin, Science, 1990, 249, 1544 CAS; (c) D. Barrick, F. M. Hughson and R. L. Baldwin, J. Mol. Biol., 1994, 237, 588 CrossRef CAS.
- H. Zemel, J. Am. Chem. Soc., 1987, 109, 1875 CrossRef CAS.
- F. W. J. Teale, Biochim. Biophys. Acta, 1959, 35, 543 CAS.
- The reaction was followed by a change in absorbance at 400 nm due to 4-nitrophenolate anion (NpO−). The pseudo first-order rate constants (kobs) were obtained according to the equation (1-[NpO−]/[Boc-Phe-ONp]0) = e−kt..
- Electronic supplementary information (ESI) available: absorbance spectra and Lineweaver–Burk plots. See http://www.rsc.org/suppdata/cc/b0/b008403o/.
- The second-order rate constant (k2) for the hydrolysis of Boc-Phe-ONp with imidazole was 23 M−1 min−1, which is much smaller than the catalytic constants (kcat/Km) for the hydrolysis of Boc-L-Phe-ONp (3.6 × 104 M−1 min−1) and Boc-D-Phe-ONp (0.24 × 104 M−1 min−1) with apomyoglobin..
- Examples of Michaelis–Menten kinetics using kobs instead of V0 in the presence of an excess amount of mediators, see (a) V. Jubian, R. P. Dixon and A. D. Hamilton, J. Am. Chem. Soc., 1992, 114, 1120 CrossRef CAS; (b) R. Hetting and H. J. Schneider, J. Am. Chem. Soc., 1997, 119, 5638 CrossRef.
- Maximum acceleration factors for the hydrolysis of the L- and D-isomers of Boc-Phe-ONp (kcat/kuncat) were 1200 and 73, respectively..
- The uncatalyzed reaction was not examined due to a limited solubility of Z-Phe-ONp..
- Boc-PhGly-ONp: kLobs = 1.3 × 10−1 min−1, kDobs = 7.1 × 10−2 min−1, kuncat = 6.4 × 10−4 min−1; Boc-Ala-ONp: kLobs = 5.1 × 10−2 min−1, kDobs = 2.7 × 10−2 min−1, kuncat = 3.1 × 10−4 min−1..
| This journal is © The Royal Society of Chemistry 2001 |
