Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Concluding remarks: a summary of the Faraday Discussion on rechargeable non-aqueous metal–oxygen batteries
Laurence J.
Hardwick

Stephenson Institute for Renewable Energy, Department of Chemistry, University of Liverpool, Peach Street, L69 7ZF Liverpool, UK. E-mail: hardwick@liverpool.ac.uk
First published on 19th December 2023
Abstract
The Faraday Discussion on rechargeable non-aqueous metal–oxygen batteries is summarised. The remarks paper highlights the specific science contributions made in the vital areas of oxygen reduction and evolution reaction mechanisms in non-aqueous electrolytes; material developments for stable metal–oxygen battery cathodes; achieving stable metal anodes and protected interfaces; and, finally, contributions concerning the progress towards practical metal–oxygen batteries. Key conclusions associated with these papers will be highlighted in an order such that readers can identify papers that they would like to explore in more detail.
Introduction
Metal–oxygen (M–O2) batteries hold the prospect of exceeding the stored energy of today's most advanced lithium-ion cells. This is not without its challenges and the field was described in Professor Janek's Concluding Remarks Lecture as “the attempt of the electrochemist to fly to the Moon or to Mars!”1Indeed, many challenges remain before M–O2 batteries could emerge as a practical technology. The recharging and cyclability efficiencies of M–O2 cells pose a major barrier to bringing this technology into practical application, as does improving our fundamental understanding of the electrochemistry and chemistry inside the cell. Understanding and controlling side reactions and the effects of reduced O2 species on all components of the cell (anode, cathode, electrolyte, separator) are not yet fully understood. Lithium–oxygen (Li–O2) and sodium–oxygen (Na–O2) are the most frequently reported types of M–O2 cells, but there has also been much recent work on K–O2, Ca–O2, and Mg–O2 systems in non-aqueous electrolytes. Ultimately, these systems present their own unique and overlapping challenges and opportunities in terms of the stability and safety of the metal electrode. Indeed, when compared to the state-of-the-art Li-ion cell, where reactions proceed via homogeneous solution-based chemistry, in all M–O2 cell types, ideal electrochemical processes involve at least two phases (heterogeneous chemistry), which presents kinetic challenges that can impact upon cycle life and rechargeability, with mitigations in cell design and engineering potentially lowering practical energy storage.
The M–O2 battery is all about oxygen redox chemistry (Fig. 1), which is one of the most important redox chemistries we have on Earth – not only important for batteries, but for life itself! Life has adapted to utilise oxygen redox and, very importantly, to survive it. Biological systems have evolved to regenerate and cope with the presence of oxygen and its other redox states. However, such controlled and stable utilisation has remained a distant dream of electrochemists, as successful replication in artificial battery systems remains in progress.
The Faraday Discussion allowed for critical examination of the state-of-play of M–O2 batteries and the identification of the major obstacles that remain in developing practical systems. Four main themes were explored in the meeting and well represented by the discussion papers: the mechanism of oxygen reduction and evolution reactions in non-aqueous electrolytes; materials for stable metal–oxygen battery cathodes; metal anodes and protected interfaces; and progress towards practical metal–oxygen batteries.
Main meeting
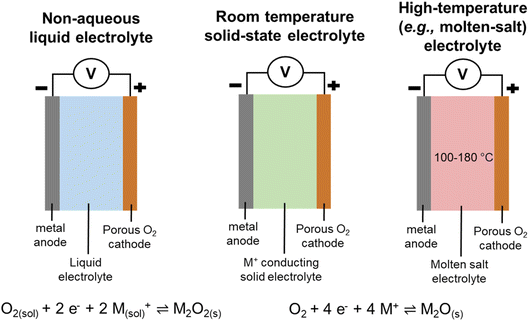
Several types of M–O2 systems were debated, including the classic non-aqueous liquid M–O2 cell, which also included investigations into the utilisation of redox mediators within these cells. In particular, the role of the I−/I3− redox mediator couple was considered in Professor Dame Clare Grey's Spiers Memorial Lecture that kick-started the discussion. The lecture also focused on methods developed to track both function and failure within M–O2 cells [https://doi.org/10.1039/D3FD00154G]. Furthermore, a recent type of room temperature all-solid-state system, and a high temperature all-solid-state cell (Fig. 2) were also deliberated.The concluding remarks are not written in chronological order, but sequenced to weave common themes within the meeting together. Furthermore, the concluding remarks for this Faraday Discussion are not intended to be a review of the field (since plenty of those already exist!) and additional citations have been only included as they were emphasised or pertinent within the discussion itself.
“Back to the future” with all-solid-state M–O2
Much work was done on M–O2 batteries before the 1990s and there are review articles that reference these pioneering works.2,3 In more recent times, the work by Abraham and Jiang4 using a polymer-electrolyte-based cell triggered a renaissance of the field. Notably, this solid electrolyte cell has inspired the recently reported polyethylene oxide (PEO)- Li10GeP2S12 Li–O2 system that utilised four-electron transformation of O2 to Li2O at room temperature from the groups of Curtiss and Asadi.5 The discussion meeting had two computational contributions led by Curtiss and his team that explored beyond their initial paper, studying the interface between Li2O and Li2O2 in this system [https://doi.org/10.1039/D3FD00083D] (Fig. 3), and the specific electrode interfaces contributing towards the templated growth of LiO2 [https://doi.org/10.1039/D3FD00116D]. These works will certainly inspire much further study, as ref. 4 did previously. Due to the nature of this complex and novel system, there are many relevant questions to be answered, leading to broad discussion of the topics, such as product identification and growth mechanisms, the epitaxial stabilisation for LiO2, Li2O2 and Li2O, the stability of the electrolyte components PEO and Li10GeP2S12, and the electrode interface/catalyst properties and behaviour of Mo3P, as well as further understanding of the hybrid-electrolyte concept.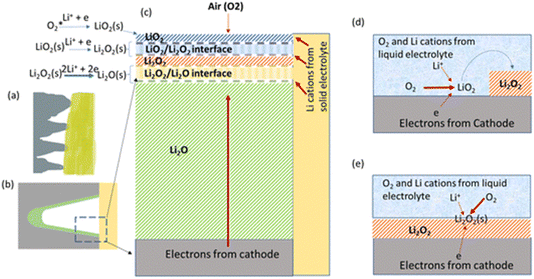 | ||
| Fig. 3 Postulated growth mechanisms for solid state Li–O2 batteries in comparison with liquid-electrolyte-based Li–O2 batteries, taken from https://doi.org/10.1039/D3FD00083D. (a) Illustration of the cathode (grey)/solid electrolyte (yellow) interface. (b) Illustration of one “valley” of the cathode (grey)/solid electrolyte (yellow) interface with a discharge deposit (light green) in the “valley”. (c) Idealised sequential reaction interphase with interfaces in a solid-state Li–O2 battery. (d) Illustration of solution-phase growth in a liquid-electrolyte-based Li–O2 battery. (e) Illustration of surface growth in a liquid-electrolyte-based Li–O2 battery. | ||
All-solid-state work was also reported by other groups, whereby the reaction pathway for Li2O generation on the surface of perovskite LaNi0.5Co0.5O3 for a molten-salt Li–O2 battery operating at 160 °C was presented and discussed [https://doi.org/10.1039/D3FD00078H]. The work and discussion highlighted that the utilisation of a molten-salt based electrolyte is another potential route towards achieving practical M–O2 cells.
Another solid-state concept was presented by Wachsman and his team on the solid-oxide fuel-cell concept of mixed ionic/electronic conductors (MIECs) for Li–O2 cells, and preliminary results were reported using a MIEC cubic garnet Li7La3Zr2O12 (LLZ) solid-state ceramic electrolyte in a 3D architecture [https://doi.org/10.1039/D3FD00119A] (Fig. 4). MIECs are materials that conduct both ions and electronic charge carriers (electrons and/or holes). The work follows on from work reported by the same team for the use of MIEC/electrolyte/MIEC architectures at the anode side to support lithium stripping and plating at high current rate density, up to 100 mA cm−2.6 The presentation generated much discussion and questions regarding product identity and growth, the achievable specific and volumetric capacities from this design approach, and the challenges relating to pore clogging and effects of side-reaction products, and will trigger considerable follow-up work.
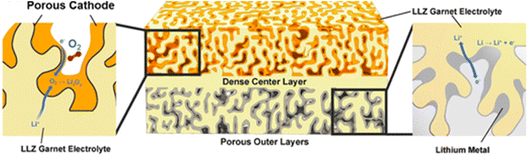 | ||
| Fig. 4 Concept of a tri-layer garnet Li7La3Zr2O12 (LLZ) Li–O2 cell with high interfacial surface area, short lithium-ion conduction pathways and minimized use of electrolyte material, taken from https://doi.org/10.1039/D3FD00119A. | ||
Liquid-electrolyte-based metal–O2 cell – “El Clásico”
A major part of the discussion was on the ‘classic’ liquid (organic/non-aqueous)-electrolyte M–O2 cell. There has been detailed work over the past 10–15 years in the study of wide ranges of liquid electrolytes. The solubility and diffusivity of O2, and the reaction intermediates, have been investigated in a wide range of systems, as well as the electrolyte effects upon the surfaces and the interfacial reactions of O2. Critically, electrolyte stability is a major challenge for non-aqueous liquid electrolytes, with much research demonstrating important degradation processes and mechanisms that impede the desired reactions for reliable rechargeable M–O2 cells. Furthermore, singlet oxygen has been identified as a highly reactive intermediate with important impacts upon stability, and the use of redox mediation and coupled redox has been demonstrated to assist in improving the kinetics of the formation and breakdown of the metal-oxide products.7,8 Many challenges remain in this area, which motivated a rigorous discussion, covering areas from the quest for stable solvents, salts and mediators, to the exact microstructure and composition of MxOy(s) discharge products, the stabilisation of metal anodes in contact with liquid electrolyte, and the development of hybrid cells with protecting solid-state or polymeric ionically conducting layers.The discussion in this topic area kicked off with work on a sustainably derived electrolyte solvent (1,2,3-trimethoxypropane) with low toxicity for sodium–O2 batteries from Ortiz-Vitoriano and coworkers [https://doi.org/10.1039/D3FD00096F]. Unlike the analogous, and toxic, diglyme solvent, the bio-based and non-toxic ether 1,2,3-trimethoxypropane showed more severe reactivity with Na-metal, owing to the nucleophilic abstraction of a proton from the tertiary carbon. The introduction of an ionic liquid, N-butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide, to the electrolyte formulation improved the stability somewhat, but the researchers demonstrated that full protection of the Na-metal interface, using a Na-ion Na-β-alumina disk, enhanced stability and cycling capacities significantly and resulted in NaO2 cubes as the primary discharge product. The work led to much discussion on the optimisation and design of stable electrolyte systems regarding the function of the ionic liquid to limit reactivity and alter reaction pathways, and the important use of a protected sodium anode using a Na-β-alumina disk as a physical barrier to separate the liquid electrolyte from the reactive interface with the sodium metal.
There were two contributions that extended our knowledge of other systems beyond Li and Na as the charge-carrying ion that focused on potassium–O2 (K–O2). The first by Wu et al [https://doi.org/10.1039/D3FD00085K] highlighted a “closed” K–O2 cell, whereby the cell is cycled via 2-electron transformation from O2 to K2O2via KO2. In the closed-cell configuration, KO2 acted as the starting active material and the work presented another possible M–O2 cell concept that will certainly motivate further investigations (Fig. 5). The second study by Peng et al [https://doi.org/10.1039/D3FD00071K] used in situ Raman spectroscopy and density functional theory to elucidate key products and intermediates (O2−, KO2 and K2O2) in the K–O2 system. These powerful model studies extend our knowledge of oxygen electrochemistry.
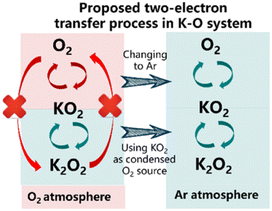 | ||
| Fig. 5 Proposed two electron process in a closed K–O2 cell system, taken from https://doi.org/10.1039/D3FD00085K. | ||
Discussion of papers that were also focused on experimental design to elucidate deeper understanding of metal–O2 systems continued. Firstly, the work by Fernandez-Vidal, Attard and co-workers [https://doi.org/10.1039/D3FD00084B] explored the effect of group 1 alkali-metal cations (Na+, K+, and Cs+) on the oxygen reduction and evolution reactions (ORR and OER). Using the method, SHell-Isolated Nanoparticle-Enhanced Raman Spectroscopy (SHINERS), reduced oxygen species could be detected at the interface of single-crystal Pt(111) electrodes. Ye and coworkers [https://doi.org/10.1039/D3FD00092C] used sum frequency generation (SFG) spectroscopy and infrared reflection absorption spectroscopy (IRRAS) to investigate the stability of a series of glyme solvents with different chain lengths, typical solvents used in M–O2 studies. The paper provided further experimental evidence that O2− generated during the ORR plays a critical role in the stability of this solvent family. Detailed mechanistic studies finished with work by Chen and colleagues on the Li–O2 system, studying the competition between the solution and surface routes of Li2O2 formation during the discharging process [https://doi.org/10.1039/D3FD00080J]. Discussions of these papers were directed on experimental design, the sensitivity of the methods, and the control and minimisation of contaminants.
Entering the quantum realm: singlet oxygen
Paraphrasing Professor Janek in his concluding remarks lecture,1 “Pity the poor battery scientists who now not only have to endure physical chemistry, but also quantum chemistry in their field.” Indeed, the hot-topic of the role and detection of singlet oxygen (1O2) in M–O2 cell chemistry was well discussed at the meeting, with two leading contributions debated. Freunberger and co-workers [https://doi.org/10.1039/D3FD00088E] showed direct spectroscopic evidence of the previously identified pathways for 1O2 formation in non-aqueous oxygen redox chemistries.7,9 Furthermore, they demonstrated that chemical trapping with 9,10-dimethylanthracene (DMA) is a consistent and reliable method to evaluate 1O2 formation (Fig. 6). Calvo and co-workers [https://doi.org/10.1039/D3FD00081H] presented the detection of 1O2 in an operational Li–O2 cell, also by DMA fluorescence decay. 1O2 was further suppressed using physical quenchers, leading to an extended cell cycle life. Discussion focused on the significance of the role of 1O2 in degradation processes within the M–O2 cell. Undoubtedly, this is a foremost area of investigation and further research directions to fully understand these issues, and their overall importance, will be actively pursued.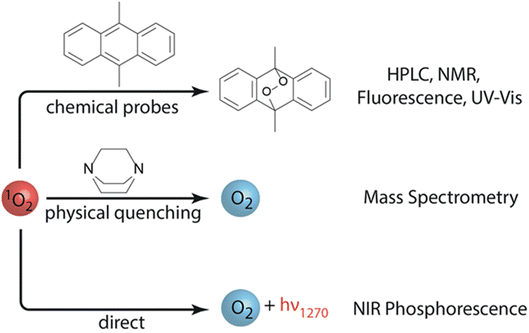 | ||
| Fig. 6 Methods to detect singlet oxygen (1O2) in non-aqueous oxygen redox systems. The use of 9,10-dimethylanthracene (DMA) as a chemical probe is highlighted. Figure taken from https://doi.org/10.1039/D3FD00088E. | ||
The mediator: “a go-between”
Redox mediators are electrolyte-soluble catalysts and, in M–O2 cells, are used to promote either the discharge or charge processes. Mediated cell operation involves an electrochemical–chemical coupled process, whereby the potential depends largely on the redox potential of the redox-mediator additive.10,11Gao, Bruce, and co-workers presented a study investigating the accumulation of lithium carbonate (Li2CO3) in a Li–O2 battery with dual mediators [https://doi.org/10.1039/D3FD00105A]. Therein, 2,5-di-tert-butyl-1,4-benzoquinone (DBBQ) and 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) were used as redox mediators in the same electrolyte formulation for the discharge and charge processes, respectively. Within this dual mediated cell, the build-up of Li2CO3 was observed. Another mediator, 2,5-di-tert-butyl-1,4-dimethoxybenzene (DBDMB), which is also capable of oxidising Li2CO3, was employed to investigate the role of Li2CO3 in cell failure and a partial recovery of the cell's performance was realised when it was also present. The work highlighted the need to avoid Li2CO3, or to have a reaction pathway available for its removal to prolong the cycle life of a Li–O2 battery. Jónsson and co-workers employed molecular dynamics to understand the electrolyte composition in the solvation of I− [https://doi.org/10.1039/D3FD00090G], which has been shown to be critical for the efficient operation of this important redox mediator. The simulations showed that the presence of water increases I−–I− interactions, which is an important requirement for I3− formation and the choice of glyme chain-length tuned these interactions further. Discussion at the meeting debated whether Li2CO3 or 1O2 was the major detrimental species and the remaining challenges in developing stable mediators. Additionally, the discussion explored the necessary requirement for protected metal anodes and the considerations that redox mediators are vital to enable practical liquid-electrolyte cells with appreciable current densities.
Protect! Protect! The study and development of stable metal anodes
The metal anode is often thought of as the forgotten child of M–O2 batteries, but nonetheless a child with as much high-spirits as the cathode side. The discussion addressed this negligence with Menkin and coworkers investigating the correlation between the shape of voltage traces and degradation in Li/Li symmetric cells [https://doi.org/10.1039/D3FD00101F]. There exists a considerable gap in performance between Li/Li symmetric cells and practical Li-metal-containing batteries. Electrochemical impedance spectroscopy (EIS) and operando NMR were used to highlight that transient (i.e., soft) short-circuits form under standard conditions for battery applications. They discussed that the typical rectangular-shaped voltage trace, widely considered ideal, was shown to be in fact a result of soft short-circuits. Furthermore, a new type of critical current density was defined in the paper: the current density at which the soft shorts are not reversible. The discussion centred around the methodology and its important future use in evaluating “shorts” in protected metal anodes. The different types of short-circuits are illustrated in Fig. 7 with the equivalent circuit for each scenario.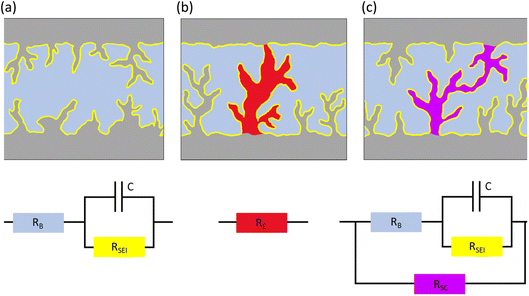 | ||
| Fig. 7 Illustrations and equivalent circuits for charge transport through the electrode–electrolyte interface in (a) purely ion transport, (b) a hard short circuit (electron transport) and (c) a soft short circuit (mixed electron and ion transport). Figure taken from https://doi.org/10.1039/D3FD00101F. | ||
The next contribution from Lee and Sakamoto focused on the generation of protected metal anodes through plating, in this case, lithium metal through a garnet Li7La3Zr2O12 (LLZ) layer onto copper current collectors (analogous to zero-excess lithium cells) [https://doi.org/10.1039/D3FD00079F]. The study reported how the stability of cycling Li anodes depends on their depth of discharge (DOD). High DOD cycling resulted in unstable cell performance from the accumulation of interfacial degradation at the Li/Li7La3Zr2O12 interface.
Another route for anode stabilisation is the in situ formation of stable ion-conductive solid interphases on lithium metal alloys. The contribution from Archer and co-workers [https://doi.org/10.1039/D3FD00112A] discussed the use of interphases composed of indium (In) for the protection of both Li and Na anodes in true metal–air configurations. These interphases were shown to protect the metal from passivation, even with operation in ambient air and, therefore, allow stable cycling to occur. Discussions focused on considering the stability of these interphases, their viability with M–O2 systems, and discharge-product characterisation.
Putting science into practice, the path to practical metal–air cells: demonstrators and performance metrics
Ultimately, to progress the field towards realistic and viable cell performance, M–O2 batteries with the requisite auxiliary components need to be assembled and tested based upon the bedrock of scientific and technological understanding. The discussion on the path to practical M–O2 cells included three papers that presented demonstrator systems, necessary engineering requirements and performance metrics, and highlighted future cell and electrolyte development.The work of Johnson and co-workers [https://doi.org/10.1039/D3FD00137G] described the assembly and testing of a Li–air battery with in-line gas handling that allows control over the flow and composition of the gas supplied to the cell. This configuration allowed for the simultaneous evaluation of the cell and scrubber performance. The cell housing and flow field is shown in Fig. 8. The test cell was able to be operated up to areal capacities of 7 mA h cm−2 at a current density of 0.5 mA cm−2. The study found that the rate of O2 flow can drastically impact the capacity of cells and further supported the requirement for utilisation of redox mediators. Additionally, the study highlighted the need for metal–air cell-specific flow-field plates and gas diffusion electrodes. Discussion focused on the description, and possible optimisation, of the gas scrubber and other auxiliary systems.
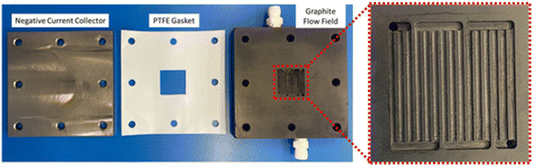 | ||
| Fig. 8 Photographs of the cell housing used in the study, with a magnified image of the flow field plate shown, as taken from https://doi.org/10.1039/D3FD00137G. | ||
Ellison and Grey considered the requirements for refining cell design and improving O2 transport, outlining expected minimum performance criteria needed to break into certain market applications (outlined in Table 1) [https://doi.org/10.1039/D3FD00091E]. Additionally, the authors also discussed the requisite parameters for an electrolyte formulation for it to be considered usable within a practical Li–air cell that could deliver a specific energy of 700 W h kg−1.
| Grid | Car | Drone | |
|---|---|---|---|
| Specific energy (W h kg−1) | — | 700+ | 700+ |
| Energy density (W h L−1) | — | 700+ | 700+ |
| Energy efficiency | 80% | 75% | 60% |
| Cycle life (to 80%) | 3000+ | 600 | 12 |
| Charge/discharge time (h) | 4/4+ | <4/4 | 12/12 |
Matsuda and colleagues presented work supported by SoftBank Corp., who are pursuing Li–O2 cell development for powering electrified aircraft [https://doi.org/10.1039/D3FD00082F]. They demonstrated that the ratio of electrolyte amount against areal capacity (E/C) has a large impact on the performance of stacked-type Li–O2 batteries. Cell degradation behaviour during the charging process under low E/C conditions was further demonstrated and the impact of differing types of gas diffusion layers on the cell performance was explored. Overall, the papers and the subsequent discussions were immensely useful in focusing in on the key parameters and, in essence, providing a roadmap to best push forward the development in the field of M–O2 batteries.
Conclusions
The metal–oxygen (M–O2) battery system is highly complex, which is why it inspires model electrochemical investigations and advanced methodological development. These developments not only advance battery chemistry, but more broadly advance chemical knowledge itself. There has been enormous progress in the fundamental understanding of Li–O2 cells throughout the past decade, which continued at this meeting. Other M–O2 batteries are being studied in parallel (M = Na, K, Si, Mg, Ca, Al), with Na–O2 and K–O2 systems being well-represented during the discussion. Performances are closing in on the requirements of practical cells, with some flavours of metal–air systems progressing towards “demonstrator system” level. Understanding the range of performance requirements, rather than targeting a single metric (i.e., just capacity), will help enormously in guiding the future research directions and framing overarching goals. Challenges remain across the field, such as redox-stable electrolytes, stable metal anodes, extent of the role of singlet oxygen, lithium carbonate accumulation, and improved mechanistic understanding and control, as well as building on progress reported on mixed ionic/electronic conductor (MIEC) cathodes and all-solid-state systems. While the scientific and technical challenges are broad, the enthusiastic deliberations from the Faraday Discussion indicated a consensus that stable rechargeable M–O2 batteries with high capacity will come, though speculation on the arrival date was deemed premature.Conflicts of interest
There are no conflicts to declare.Acknowledgements
LJH acknowledges funding support from the UK Faraday Institution (EPSRC EP/S003053/1) through the Degradation Project (grant numbers FIRG001 and FIRG024) and is extremely grateful to Thukshan Samarakoon and Dr Alex Neale for creation of Fig. 2 and scientific discussions on the manuscript. Sections of the manuscript were inspired from notes taken during Professor Juergen Janek's stimulating closing concluding remarks lecture.Notes and references
- J. Janek, Concluding remarks lecture – rechargeable non-aqueous M–O2 batteries, 2023.
- K. F. Blurton and A. F. Sammells, J. Power Sources, 1979, 4, 263–279 CrossRef CAS.
- T. Liu, J. P. Vivek, E. W. Zhao, J. Lei, N. Garcia-Araez and C. P. Grey, Chem. Rev., 2020, 120(14), 6558–6625 CrossRef CAS.
- K. M. Abraham and Z. Jiang, J. Electrochem. Soc., 1996, 143, 1–5 CrossRef CAS.
- A. Kondori, et al. , Science, 2023, 379, 499–505 CrossRef CAS PubMed.
- G. V. Alexander, C. Shi, J. O'Neill and E. D. Wachsman, Nat. Mater., 2023, 22, 1136–1143 CrossRef CAS.
- Y. K. Petit, E. Mourad and C. Prehal, et al. , Nat. Chem., 2021, 13, 465–471 CrossRef CAS.
- A. Schürmann, B. Luerßen, D. Mollenhauer, J. Janek and D. Schröder, Chem. Rev., 2021, 121, 12445–12464 CrossRef PubMed.
- N. Mahne, B. Schafzahl and C. Leypold, et al. , Nat. Energy, 2017, 2, 17036 CrossRef CAS.
- Y. Chen, Y. S. Freunberger and Z. Peng, et al. , Nat. Chem., 2013, 5, 489–494 CrossRef CAS.
- B. J. Bergner, A. Schürmann, K. Peppler, A. Garsuch and J. Janek, J. Am. Chem. Soc., 2014, 136, 15054–15064 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |