Molecular logic operations from complex coacervation with aggregation-induced emission characteristics†
Jianhui
Liu
 a,
Tianfu
Zhang
a,
Tianfu
Zhang
 b,
Xiaolin
Liu
b,
Xiaolin
Liu
 b,
Jacky W. Y.
Lam
b,
Ben Zhong
Tang
b,
Jacky W. Y.
Lam
b,
Ben Zhong
Tang
 bc and
Ying
Chau
bc and
Ying
Chau
 *a
*a
aDepartment of Chemical and Biological Engineering, the Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China. E-mail: keychau@ust.hk
bDepartment of Chemistry and Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, the Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China
cSchool of Science and Engineering, Shenzhen Key Laboratory of Functional Aggregate Materials, the Chinese University of Hong Kong, Shenzhen, Guangdong 518172, China
First published on 6th July 2022
Abstract
Leveraging complex coacervation of a polycation and a bivalent anion with aggregation-induced emission characteristics, we accomplish eight basic logic operations with environmental stimuli as inputs, producing Boolean-like fluorescence intensity or turbidity ‘outputs’ with contrast higher than one order of magnitude. Storage of information of a fluorescent pattern and thermo-sensor applications are also demonstrated.
New conceptsMolecular logic operations have been largely constructed as they exemplify the miniaturization of Boolean operations. However, most of the reported systems only performed limited types of basic logic operations or/and exhibited limited contrast between Boolean output signals. Here, simply exploiting the complex coacervation between a polycation and a bivalent anion with aggregation-induced emission characteristics, we accomplish all the eight basic logic operations, namely, ‘Buffer’, ‘NOT’, ‘AND’, ‘OR’, ‘NAND’, ‘NOR’, ‘XOR’ and ‘XNOR’, producing Boolean-like fluorescence intensity or turbidity ‘outputs’ with contrast higher than one order of magnitude. Moreover, a matrix of Buffer-type molecular logic gates is fabricated to store the information of a fluorescent pattern, and a solidified NOT-type logic gate in a hydrogel is exploited as a thermo-sensor of the surrounding solution. This work can help to boost the design of simple yet high-contrast molecular logic gates, together with the development of information storage media and environmental sensor devices. |
Molecular logic operations are performed via molecular logic gates based on the ‘digital’ molecular response to changes in the environment,1–3 wherein one or more binary inputs produce a single binary output.4 Molecular logic gates have been constructed and investigated owing to their fundamental roles in the integration, processing and storage of information, as well as a wide array of applications spanning biosensors, environmental monitoring, and diagnosis of diseases.1 A wide array of materials have been used as the building blocks of molecular logic gates, including small molecules,5–7 polymers,8 coordination materials9 and biological materials,10,11 which can be triggered by inputs with distinctive nature, including physical, chemical and biological stimuli.12 In particular, the eight basic logic operations with single input (‘Buffer’13–19 and ‘NOT’16–18) or two inputs (‘AND’,5,13–16,19–24 ‘OR’,13–15,17,22,23,25 ‘NAND’,5,22–24,26,27 ‘NOR’,17,22,23 ‘XOR’15,20,28,29 and ‘XNOR’28,30–33) have aroused broad interest. To the best of our knowledge, these reports perform limited basic logic operations,23,24,27,34 require complicated fabrication processes,15,23 or exhibit limited contrast between outputs.15,23,24,27,35 It will be promising if a concise and easy-to-make molecular system can achieve all basic molecular operations with high contrast between Boolean outputs.
Fluorescence has been widely used as an optical output of molecular logic operations owing to the ease of observation.12,36 The well-known quenching effect of luminophores causes a decrease of fluorescence intensity (FI), affording the intrinsic nature of a NOT logic operation. Distinctively, aggregation-induced emission (AIE) is an unconventional photophysical phenomenon coined in 2001.37–41 The AIE luminogens are non-emissive under the dispersed state; however, they become emissive under conditions of aggregation or restriction of intramolecular motion, and have been developed into smart materials40 responsive to multifaceted stimuli such as mechanics, temperature, pH and light.39 Coacervation, also known as liquid–liquid phase separation,42 is a pivotal43–45 way of intracellular organization of biomolecules and is fundamental to the formation of membraneless organelles.46–50 The peculiar liquidity of membraneless organelles affords cells to host compartments with high dynamics, mobility, environmental responsiveness and reversibility.51 Complex coacervation is the process of associative phase separation of oppositely-charged molecular species, generating a condensed phase (coacervate) and a dilute phase (dispersed phase).52 We reason that complex coacervation with AIE luminogens (AIEgens) that otherwise do not self-aggregate can trigger prominent and environment-responsive enrichment of the dye. This would lead to restriction of the intramolecular motion of AIEgens and allow the excitons to decay radiatively. In this paper, leveraging a binary complex coacervation phenomenon comprising AIEgens, we demonstrate the construction of eight basic molecular logic operations with FI or turbidity as the digital-like Boolean outputs.
Polyelectrolytes can undergo complex coacervation with charge-complementary species,52–54 particularly with counterionic bivalent small molecules as transient and weak ‘crosslinkers’ to mediate complex coacervation.55 The arginine–glycine–glycine motif is widely presented in human proteins for molecular interactions including electrostatic attraction.56 As such, we grafted dextran57 with cysteine-terminated (arginine–glycine–glycine)-containing peptide (Ac–CGGRGG–CONH2), to synthesize a dextran-CGGRGG hybrid (DCH) as the polycation (Fig. 1). For the counterionic partners, we synthesized 1,2-bis[4-(4-sulfonatobutoxyl)phenyl]-1,2-diphenylethene salt (BSBOTPE, Fig. 1), a bivalent, anionic and hydrophilic small molecule comprising tetraphenylethene with AIE characteristics.58 Whilst the DCH or BSBOTPE alone remained dispersed in solution, the coexistence of DCH and BSBOTPE engendered coacervation in a stoichiometry-dependent and temperature-responsive manner with an upper critical solution temperature (UCST), as indicated by turbidimetry (Fig. S1A and C, ESI†). This UCST-type59 phase behavior is consistent with a previous report of complex coacervation comprising hydrophilic polyelectrolytes.60,61 Moreover, the presence of coacervation is concomitant with the surge of fluorescence in a coherent fashion (Fig. S1B and D, ESI†), as indicated by overall FI. These data suggest that the complex coacervation phenomenon could be exploited for molecular logic operations with environmental stimuli and chemicals as ‘inputs’, and FI or turbidity as ‘outputs’ with high contrast between ‘0’ and ‘1’ values.
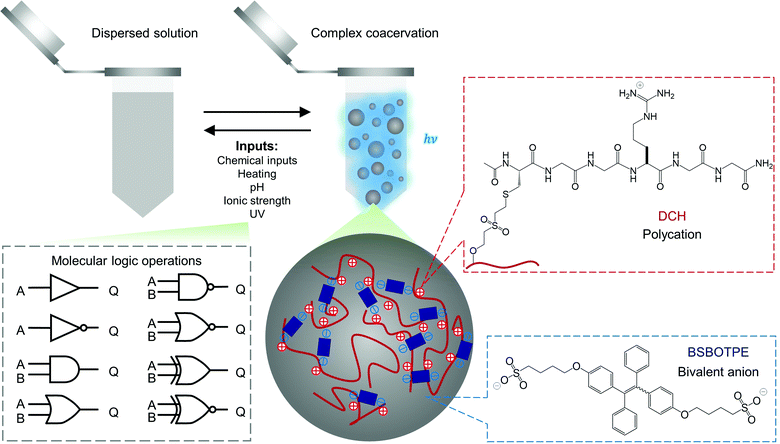 | ||
| Fig. 1 Schematic illustration of complex coacervation and application for molecular logic operations thereof. | ||
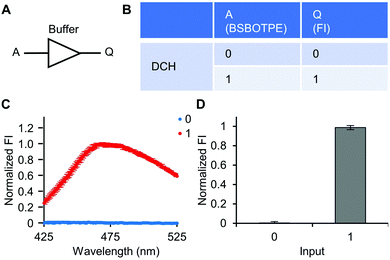
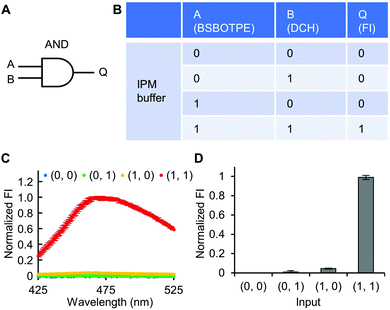
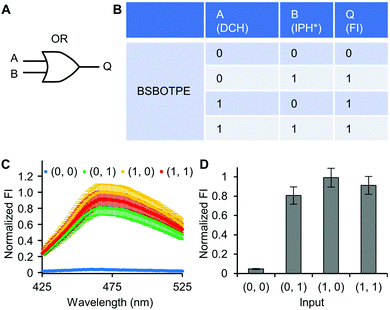
Firstly, DCH and BSBOTPE were used as inputs to demonstrate Buffer and AND logic operations. The relative FI at 471 nm is viewed as the output with 0.5 as the threshold value. For a Buffer logic operation, the output (‘0’ or ‘1’) is the same as the input (‘0’ or ‘1’) (Fig. 2A and B). DCH alone was non-fluorescent (output = 0), whilst the addition of BSBOTPE generated complex coacervation and aggregation of BSBOTPE in coacervate phase, thereby producing high FI signal (output = 1) (Fig. 2C and D). The role of DCH and BSBOTPE can be switched to perform another Buffer logic operation (Fig. S2, ESI†). For an AND logic gate, the output is ‘1’ only if both inputs are ‘1’ (Fig. 3A and B). This scenario is well represented by the combination of BSBOTPE and DCH as the inputs, namely, only the coexistence thereof can generate ‘1’ output owing to complex-coacervation-caused AIE (Fig. 3C and D). For an OR logic gate, the output is ‘1’ if either of the inputs is ‘1’ (Fig. 4A and B). We added an intrinsically disordered protein (IDP)-mimicking polymer-oligopeptide hybrid* (IPH*), which is capable of self-coacervation and recruitment of charged cargoes,62–64 as an input. Therefore, we used DCH and IPH*, the macromolecules without and with self-coacervation capability, respectively, as the two inputs to the BSTOPE gate. Incorporation of DCH or IPH* or both exhibited coacervation in complexation with BSBOTPE to generate high FI (output = 1) (Fig. 4C and D). All FI data of Buffer, AND and OR logic operations are consistent with turbidimetry (Fig. S3A–D, ESI†) and confocal laser scanning microscopy (CLSM) (Fig. S4–S7, ESI†).
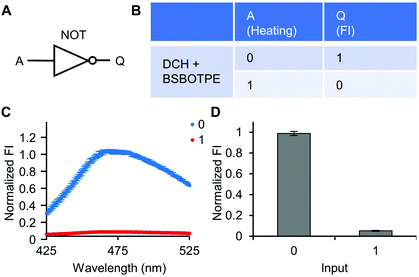
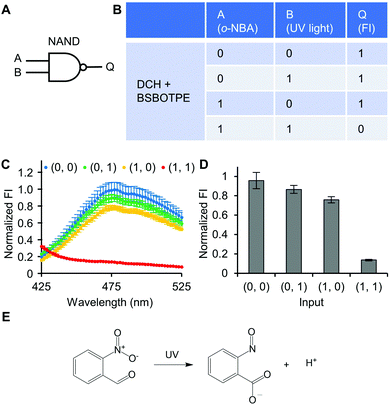
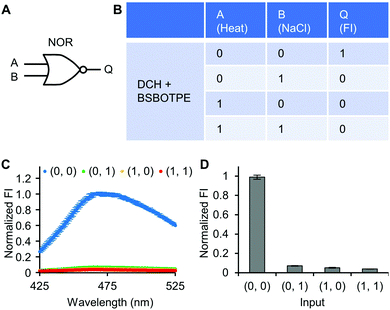
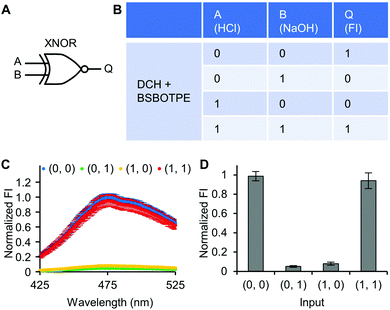
Next, pre-formed complex coacervates from ‘DCH + BSBOTPE’ were used as the gate to demonstrate NOT, NAND, NOR and XNOR logic operations. The relative FI at 471 nm is viewed as the output with 0.5 as the threshold value. For a NOT logic operation, the output (‘0’ or ‘1’) is different from the input (‘0’ or ‘1’) (Fig. 5A and B). The complex coacervation from DCH and BSBOTPE exhibited high FI signal (output = 1), whilst the employment of mild heating (from 25 °C to 40 °C) dissolved coacervates and reversed the aggregation of BSBOTPE to the dispersed state, thus producing a low FI signal (output = 0) (Fig. 5C and D). Salt can be used as another ‘input’ to perform NOT logic operation (Fig. S8, ESI†) owing to a charge screening65 effect. For a NAND logic gate, the output is ‘0’ only if both inputs are ‘1’ (Fig. 6A and B). The o-NBA is a photoacid capable of releasing protons upon UV irradiation,66 thereby engendering the drop of pH67 (Fig. 6E). Whilst the o-NBA or UV light treatment alone had negligible effect on the FI (output = 1), the concurrence thereof triggered a pH drop and protonation of BSBOTPE, thereby disrupting the charge complementary complex coacervation and AIE to produce low FI (output = 0) (Fig. 6C and D). For a NOR logic gate, the output is ‘1’ only if both inputs are ‘0’ (Fig. 7A and B). Heat or salt or both can dissolve the complex coacervation and reverse the aggregation of BSBOTPE to the dispersed state, thereby producing low FI signal (output = 0) (Fig. 7C and D). For a XNOR logic gate, the output is ‘1’ only if the inputs are the same, namely, both are ‘0’ or ‘1’ (Fig. 8A and B). The complex coacervation between DCH and BSBOTPE produces high FI without input (output = 1). With the addition of HCl or NaOH, the protonation of BSBOTPE or the deprotonation of DCH was engendered, respectively, thereby eliminating the charge–complementary complex coacervation and aggregation of BSBOTPE (output = 0). With the addition of both HCl and NaOH, neutralization thereof produced marginal effect (output = 1) (Fig. 8C and D). For NOT, NAND and NOR logic operations, FI data are consistent with turbidimetry (Fig. S3E–H, ESI†) and CLSM (Fig. S9–S12, ESI†). Note that for XNOR logic operation, the FI data are consistent with CLSM (Fig. S13, ESI†), whilst there is a mismatch of turbidimetry and FI with the input (0, 1) (Fig. S3I, ESI†), namely, the FI signal is low, whilst the turbidity is high. This surge of turbidity is attributed to the self-coacervation of DCH under alkaline conditions (Fig. S3K, ESI†), which generates little complex coacervation and aggregation of BSBOTPE, resulting in low FI (output = 0).
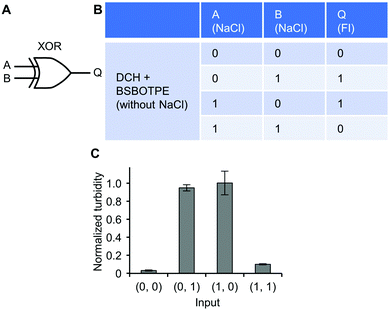
Further, a mixture of ‘DCH + BSBOTPE’ was used as the gate to demonstrate XOR logic operations, wherein the relative turbidity is viewed as the output with 0.5 as the threshold value. For a XOR logic gate, the output is ‘0’ only if the inputs are the same, namely, both are ‘0’ or ‘1’ (Fig. 9A and B). Without input, there is only prominent formation of sub-micron-sized droplets, thus resulting in little scattering of visible light and no turbidity (Fig. 9C and Fig. S3J and S14, ESI†) (output = 0). With the input of NaCl to adjust [NaCl] to an intermediate level (1200 mM), there is prominent formation of micron-sized droplets, thus producing extremely high turbidity (Fig. 9C and Fig. S3J and S14, ESI†) (output = 1). With the input of twofold NaCl to adjust [NaCl] to 2400 mM, however, this high ionic strength generates a significant charge screening effect to melt complex coacervation, thereby producing low turbidity (Fig. 9C and Fig. S3J, S14, ESI†) (output = 0). Note that the amount of turbidity does not totally match that of the FI. The low-turbidity-generating input (0, 0) results in high FI, owing to the complex coacervation on the sub-micron scale (Fig. S14 and S15, ESI†). The high-turbidity-generating inputs (0, 1) and (1, 0) result in low FI, owing to the blockage of light transmittance in the bulk solution environment with extremely high turbidity (Fig. S3J, S14 and S15, ESI†), albeit some fluorescence can be seen via CLSM (Fig. S14, ESI†).
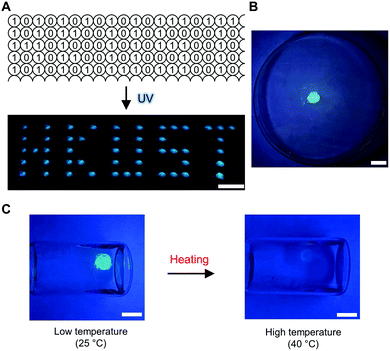
Lastly, we sought to demonstrate some proof-of-concept applications that employ these molecular logic operations for the further development of organic luminescent materials in aggregation.68 For information storage and read-out, for example, we applied the ‘Buffer (2)’ logic operations. A matrix of BSBOTPE (15 μL each, as the gate) was pre-loaded into a 384-well plate, followed by DCH (15 μL each, as the input) loading as a pattern of letters ‘HKUST’. This is the information storage process and no pattern can be seen by the naked eye. Upon UV irradiation for information reading, the wells with DCH input emitted intense blue light, resulting into a ‘HKUST’-like fluorescent pattern with high signal-to-background ratio (Fig. 10A). To construct a thermo-sensor, for example, we used the solution of complex coacervates from ‘DCH + BSBOTPE’ as the solvent to prepare dextran hydrogels crosslinked by a vinylsulfone–thiol Michael addition reaction,57 thereby achieving the ‘solidification’ of solution-state logic gates (Fig. 10B and ESI†). A solidified ‘NOT’ logic gate was leveraged for temperature monitoring of the sample solution, whilst the ‘output’ can be easily acquired by the naked eye with the assistance of a portable UV lamp (Fig. 10C).
Conclusions
Leveraging the complex coacervation of polycationic DCH and anionic AIEgen named BSBOTPE, we demonstrated eight basic molecular logic operations with high contrast between Boolean outputs. Environmental stimuli, including heating, pH, ionic strength, ultraviolet light and chemicals (including polycation, IDP-mimetic, bivalent anion and photoacid) were used as either inputs or gates, whilst FI and turbidity were outputs. This work represents a simple yet versatile platform for the execution of molecular logic operations with high contrast between outputs, which can pave the way to the performance of integrated and complicated Boolean operations on the molecular level, which support applications such as information storage and readout media and environmental sensor devices.Author contributions
Conceptualization: J. L.; investigation: J. L., T. Z. and X. L.; supervision: J. W. Y. L., B. Z. T. and Y. C.; funding acquisition: B. Z. T. and Y. C.; writing – original draft: J. L.; writing – review and editing: J. L., T. Z., X. L., J. W. Y. L., B. Z. T. and Y. C.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support was provided by the Hong Kong Research Grant Council (GRF 16102520 and 16103517) and the Innovation and Technology Commission (ITC CNERC14SC01).Notes and references
- L. Liu, P. Liu, L. Ga and J. Ai, ACS Omega, 2021, 6, 30189–30204 CrossRef CAS PubMed.
- A. P. de Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399–410 CrossRef CAS PubMed.
- J. Andréasson and U. Pischel, Chem. Soc. Rev., 2015, 44, 1053–1069 RSC.
- A. Muthukrishnan and S. Stroud, Phys. Rev. A: At., Mol., Opt. Phys., 2000, 62, 052309 CrossRef.
- X. Cao, X. Zeng, L. Mu, Y. Chen, R. X. Wang, Y. Q. Zhang, J. X. Zhang and G. Wei, Sens. Actuators, B, 2013, 177, 493–499 CrossRef CAS.
- K. L. Kompa and R. D. Levine, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 410–414 CrossRef CAS PubMed.
- A. P. de Silva, S. Uchiyama, T. P. Vance and B. Wannalerse, Coord. Chem. Rev., 2007, 251, 1623–1632 CrossRef.
- S. Uchiyama, N. Kawai, A. P. De Silva and K. Iwai, J. Am. Chem. Soc., 2004, 126, 3032–3033 CrossRef CAS PubMed.
- F. Pu, E. Ju, J. Ren and X. Qu, Adv. Mater., 2014, 26, 1111–1117 CrossRef CAS PubMed.
- D. Woods, D. Doty, C. Myhrvold, J. Hui, F. Zhou, P. Yin and E. Winfree, Nature, 2019, 567, 366–372 CrossRef CAS PubMed.
- C. Mao, T. H. LaBean, J. H. Reif and N. C. Seeman, Nature, 2000, 407, 493–496 CrossRef CAS PubMed.
- A. P. de Silva, Molecular Logic-based Computation, The Royal Society of Chemistry, 2012 Search PubMed.
- N. Cheng, P. Zhu, Y. Xu, K. Huang, Y. Luo, Z. Yang and W. Xu, Biosens. Bioelectron., 2016, 84, 1–6 CrossRef CAS PubMed.
- A. Bader and S. L. Cockroft, Chem. – Eur. J., 2018, 24, 4820–4824 CrossRef CAS PubMed.
- X. Lin, Y. Liu, J. Deng, Y. Lyu, P. Qian, Y. Li and S. Wang, Chem. Sci., 2018, 9, 1774–1781 RSC.
- M. Kluciar, R. Ferreira, B. De Castro and U. Pischel, J. Org. Chem., 2008, 73, 6079–6085 CrossRef CAS PubMed.
- Y. Shiraishi, Y. Tokitoh and T. Hirai, Chem. Commun., 2005, 5316–5318 RSC.
- A. P. De Silva, M. R. James, B. O. F. McKinney, D. A. Pears and S. M. Weir, Nat. Mater., 2006, 5, 787–790 CrossRef PubMed.
- J. F. Callan, A. P. De Silva and N. D. McClenaghan, Chem. Commun., 2004, 2048–2049 RSC.
- S. Kou, N. L. Han, D. Van Noort, K. M. K. Swamy, H. K. So, H. S. Jung, K. M. Lee, S. W. Nam, J. Yoon and S. Park, Angew. Chem., Int. Ed., 2008, 47, 872–876 CrossRef CAS PubMed.
- M. Privman, T. K. Tarn, M. Pita and E. Katz, J. Am. Chem. Soc., 2009, 131, 1314–1321 CrossRef CAS PubMed.
- J. Chen, J. Pan and C. Liu, Anal. Chem., 2020, 92, 6173–6180 CrossRef CAS PubMed.
- M. Massey, I. L. Medintz, M. G. Ancona and W. R. Algar, ACS Sens., 2017, 2, 1205–1214 CrossRef CAS PubMed.
- A. Saghatelian, N. H. Völcker, K. M. Guckian, V. S. Y. Lin and M. R. Ghadiri, J. Am. Chem. Soc., 2003, 125, 346–347 CrossRef CAS PubMed.
- L. Wang, Y. Zhang and Y. Dong, Sensors, 2018, 18, 3280 CrossRef PubMed.
- Y. Ma, X. Jin, Y. Xing, G. Ni and J. Peng, Anal. Methods, 2019, 11, 2033–2040 RSC.
- H. Liu, Y. Zhou, Y. Yang, W. Wang, L. Qu, C. Chen, D. Liu, D. Zhang and D. Zhu, J. Phys. Chem. B, 2008, 112, 6893–6896 CrossRef CAS PubMed.
- A. Coskun, E. Deniz and E. U. Akkaya, Org. Lett., 2005, 7, 5187–5189 CrossRef CAS PubMed.
- S. Wang, J. Sun, J. Zhao, S. Lu and X. Yang, Anal. Chem., 2018, 90, 3437–3442 CrossRef CAS PubMed.
- M. Asakawa, P. R. Ashton, V. Balzani, A. Credi, G. Mattersteig, O. A. Matthews, M. Montalti, N. Spencer, J. F. Stoddart and M. Venturi, Chem. – Eur. J., 1997, 3, 1992–1996 CrossRef CAS.
- M. Kumar, R. Kumar and V. Bhalla, Tetrahedron Lett., 2010, 51, 5559–5562 CrossRef CAS.
- M. Schmittel, P. Mal and A. De Los Rios, Chem. Commun., 2010, 46, 2031–2033 RSC.
- M. Zhou, F. Wang and S. Dong, Electrochim. Acta, 2011, 56, 4112–4118 CrossRef CAS.
- A. Prasanna De Silva and N. D. McClenaghan, J. Am. Chem. Soc., 2000, 122, 3965–3966 CrossRef.
- D. Gust, T. A. Moore and A. L. Moore, Chem. Commun., 2006, 1169–1178 RSC.
- P. A. De Silva, N. H. Q. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42–44 CrossRef.
- J. Luo, Z. Xie, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- H. Wang, E. Zhao, J. W. Y. Lam and B. Z. Tang, Mater. Today, 2015, 18, 365–377 CrossRef CAS.
- S. Deshpande, F. Brandenburg, A. Lau, M. G. F. Last, W. K. Spoelstra, L. Reese, S. Wunnava, M. Dogterom and C. Dekker, Nat. Commun., 2019, 10, 1–11 CrossRef CAS PubMed.
- C. P. Brangwynne, C. R. Eckmann, D. S. Courson, A. Rybarska, C. Hoege, J. Gharakhani, F. Jülicher and A. A. Hyman, Science, 2009, 324, 1729–1732 CrossRef CAS PubMed.
- S. F. Banani, H. O. Lee, A. A. Hyman and M. K. Rosen, Nat. Rev. Mol. Cell Biol., 2017, 18, 285–298 CrossRef CAS PubMed.
- J. Liu, F. Zhorabek and Y. Chau, arXiv Prepr. arXiv2104.10927.
- A. A. Hyman, C. A. Weber and F. Jülicher, Annu. Rev. Cell Dev. Biol., 2014, 30, 39–58 CrossRef CAS PubMed.
- S. Alberti, A. Gladfelter and T. Mittag, Cell, 2019, 176, 419–434 CrossRef CAS PubMed.
- H. Wu and M. Fuxreiter, Cell, 2016, 165, 1055–1066 CrossRef CAS PubMed.
- J. A. Riback, L. Zhu, M. C. Ferrolino, M. Tolbert, D. M. Mitrea, D. W. Sanders, M.-T. Wei, R. W. Kriwacki and C. P. Brangwynne, Nature, 2020, 581, 209–214 CrossRef CAS PubMed.
- J. Liu, R. Feng and Y. Chau, Matter, 2022, 5, 1637–1639 CrossRef.
- Y. Shin and C. P. Brangwynne, Science, 2017, 357, eaaf4382 CrossRef PubMed.
- C. E. Sing and S. L. Perry, Soft Matter, 2020, 16, 2885–2914 RSC.
- C. S. Cummings and A. C. Obermeyer, Biochemistry, 2018, 57, 314–323 CrossRef CAS PubMed.
- E. Kizilay, A. B. Kayitmazer and P. L. Dubin, Adv. Colloid Interface Sci., 2011, 167, 24–37 CrossRef CAS PubMed.
- W. M. Babinchak, B. K. Dumm, S. Venus, S. Boyko, A. A. Putnam, E. Jankowsky and W. K. Surewicz, Nat. Commun., 2020, 11, 1–15 CrossRef PubMed.
- P. Thandapani, T. R. O’Connor, T. L. Bailey and S. Richard, Mol. Cell, 2013, 50, 613–623 CrossRef CAS PubMed.
- J. Liu, R. Ni and Y. Chau, Chem. Commun., 2019, 55, 7093–7096 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC.
- K. Reiche, J. Hartl, A. Blume and P. Garidel, Biophys. Chem., 2017, 220, 7–19 CrossRef CAS PubMed.
- H. Kim, B. Jin Jeon, S. Kim, Y. S. Jho and D. S. Hwang, Polymers, 2019, 11, 691 CrossRef CAS PubMed.
- T. Maji, S. Banerjee, Y. Biswas and T. K. Mandal, Macromolecules, 2015, 48, 4957–4966 CrossRef CAS.
- J. Liu, F. Zhorabek, X. Dai, J. Huang and Y. Chau, ACS Cent. Sci., 2022, 8, 493–500 CrossRef CAS PubMed.
- J. Liu, F. Zhorabek and Y. Chau, ACS Macro Lett., 2022, 32, 562–567 CrossRef PubMed.
- J. Liu, F. Zhorabek, T. Zhang, J. W. Y. Lam, B. Z. Tang and Y. Chau, Small, 2022, 18, 2201721 CrossRef CAS PubMed.
- C. D. Keating, Acc. Chem. Res., 2012, 45, 2114–2124 CrossRef CAS PubMed.
- J. M. Allen, S. K. Allen and S. W. Baertschi, J. Pharm. Biomed. Anal., 2000, 24, 167–178 CrossRef CAS PubMed.
- Y. Gao and M. J. Serpe, ACS Appl. Mater. Interfaces, 2014, 6, 8461–8466 CrossRef CAS PubMed.
- J. Yang, M. Fang and Z. Li, Aggregate, 2020, 1, 6–18 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, methods, supplementary turbidity data, fluorescence intensity data and confocal laser scanning microscopy. See DOI: https://doi.org/10.1039/d2mh00537a |
| This journal is © The Royal Society of Chemistry 2022 |