Open Access Article
Open Access ArticleTowards conductive hydrogels in e-skins: a review on rational design and recent developments
Chujia Li *
*
Queen Mary University of London Engineering School, Northwestern Polytechnical University, Xi'an, Shaanxi Province 710072, China. E-mail: chujiali@mail.nwpu.edu.cn
First published on 18th October 2021
Abstract
Over the past decades, electronic skins (e-skins) have attracted significant attention owing to their feasibility of applications in health monitoring, motion detection, and entertainment. As a class of soft materials, conductive hydrogels feature biocompatibility, stretchability, adhesiveness, and self-healing properties, making them one of the most important candidates for high-performance e-skins. However, profound challenges remain for achieving the combination of superior mechanical strength and conductivity of conductive hydrogels simultaneously without sacrificing their multifunctionalities. Herein, a framework for rational designs to fabricate conductive hydrogels are proposed, including the fundamental strategies of copolymerization, doping, and self-assembly. In addition, we provide a comprehensive analysis of their merits and demerits when the conductive hydrogels are fabricated in different ways. Furthermore, the recent progress and future perspective for conductive hydrogels in terms of electronic skins are highlighted.
1 Introduction
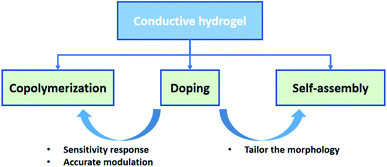
Electronic skins (e-skins) have become an attractive topic in modern society since they brought utmost convenience to monitor the pulse, heartbeats, motion, and other activities of human beings.1–4 Owing to the responsive properties, the circuit systems of e-skins can transform the environmental stimuli (e.g., temperature, pressure, strain, and light) into electrical signals and give appropriate responses, shining a light on the usage in biomedical and energy storage industries.3,5–10 So far, many works on fabricating e-skins have focused on inorganic materials, including graphene,11–13 semiconductors,14–18 and alginate-based materials.19 Although they exhibit outstanding conductivity and mechanical properties, it remains a challenge to achieve softness, self-healing properties, and biocompatibility, which are critical factors for a high-performance e-skin.20,21 Conductive hydrogel is one of the most popular alternative candidates to tackle these issues by virtue of the excellent electrical conductivity and flexibility,22–25 which highly matches the demands for fabricating flexible electronics, especially the critical conditions of e-skins.Recent advances of conductive hydrogels have attracted a lot of attention on e-skins owing to their stretchability,16–18 sensitivity,28,29 self-repairing capacity,30–32 flexibility,26,27,33 and excellent compatibility.34–36 However, in practical applications of e-skins, traditional hydrogels are not robust enough to sustain cyclic loading and impact strength.6 There is an urgent need to improve the mechanical strength and conductivity of hydrogels without impairing their own features, such as stimulus-responsive compatibility, adhesiveness, and biocompatibility. Various synthesis methodologies have been proposed to develop advanced conductive hydrogels to address such challenges, including copolymerization, doping, and self-assembly (Fig. 1).23,37,38
 | ||
| Fig. 1 Illustration of structural designs and methodologies to fabricate conductive hydrogels in the application of e-skins. | ||
Among such important techniques, copolymerization is an effective and straightforward strategy to modulate the proportions of different monomers via direct copolymerization reaction,39 or modify the functional groups to realize specific properties and introduce crosslink networks by grafting.40–42 Representative systems for copolymerization are conductive polymers include polyaniline (PANI) and polypyrrole (PPy), which are attributed to their excellent conductivity and biocompatibility, or hydrophilic chains including poly(N-vinylpyrrolidone), poly(ethylene glycol), and polyacrylic acid, to endow the hydrogels with self-healing and adhesiveness.23,41–43 Such strategy is easy to be processed, but a serious concern is that the conductive stability and mechanical strength (i.e., in the range of tissues and organs) of hydrogels are difficult to be accurately controlled since these properties are tightly dependent on the amounts and features of each monomer.23
Consequently, it is arduous to achieve accurate control during the polymerization process. To tackle this issue, doping is selected as a facile method to adjust the conductivity and mechanical strength of hydrogels directly by simply incorporating conductive charges, acids, or polymer chains.26,33,37,44–48 Also, the stability of the system can be enhanced by forming gelated nanostructures in hydrogels.37,49 It is generally accepted that the conductive PPy, PANI, and phytic acid (PA) can be doped into the hydrogel structure to enhance the conductivity, and the porous microstructure or crosslinking network can be generated to endow the hydrogels with high stretchability.50,51
Furthermore, self-assembly is regarded as another novel and advanced strategy to fabricate conductive hydrogels with self-healing properties due to the existence of weaker interactions among polymer chains, including p–p stacking,52–55 van der Waals forces,56 hydrophobic interactions,57,58 hydrogen bonding,59 and electrostatic interactions,60,61 which all effectively drive the formation of hydrogels. However, it should be noted that the stability of self-assembled hydrogels is still limited because they may largely be influenced by the external environment.62,63 Thus, further effort is required to develop a hydrogel system that is sufficiently stable and efficient for practical use in e-skins.
Herein, we propose a theoretical framework of methodologies to summarize the recent progress in high-performance conductive hydrogels for flexible electronics and further provide novel insights about the rational design of advanced hydrogels based on their structural designs and the chemical interactions in the polymer networks. We review the feasible methodologies of synthesizing conductive hydrogels, present the major advantages for each approach, and highlight their bright perspectives in the applications of flexible electronic devices, especially for their utilization and great advantages for e-skins. This review aims to construct a clear theoretical framework to illustrate the main fabrication approaches for conductive hydrogels and provide novel insights to develop the advanced strategies of hydrogel fabrication, while their promising future on the potential usage of e-skins is further clarified and discussed.
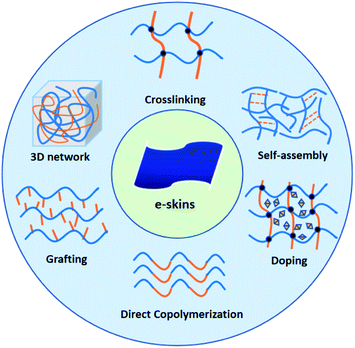
2 Copolymerization
Copolymerization reaction is a quintessential methodology to generate conductive hydrogels by combining the unique properties in different types of monomers, polymer chains, or components.64,65 Among all the copolymerization strategies, direct copolymerization and grafting are two widely utilized approaches to fabricate conductive hydrogels through copolymerization, realizing multifunctional features of hydrogels, such as conductivity, stretchability, stability, self-healing property, and biocompatibility.34,35,66–81 However, the majority of hydrogels are restricted by the poor mechanical strength,82 leading to insufficient stability and efficiency for practical utilization. To tackle the limitations on structural robustness, incorporation of high-strength fillers and constructing crosslinked structures are designed during fabrication.34,70,71,78,80 By optimizing conductive hydrogels featuring superior strength and conductivity, they are of paramount importance in the applications of e-skins or wearable electronics.2.1 Direct copolymerization
Direct copolymerization is of great significance to fabricate conductive hydrogels by simply mixing monomers with different ratios or adding them into hydrogel precursors, followed by feeding a gelator or changing the external environments to activate the reaction.83 These conductive hydrogels can be applied to generate multifunctional e-skins or flexible electronic devices.66–73 A facile methodology of direct copolymerization for conductive hydrogels is to control the content of monomers in the copolymers in order to realize the specific properties of hydrogels. For example, Jiang et al. established a mechanical-tunable conductive hydrogel by copolymerizing hydrophobic HEMA (hydroxyethyl methacrylate) and AA (acrylic acid), catering to the different requirements of mechanical properties of hydrogels.66 Owing to the adjustable mechanical and conductive capacity,66 such a hydrogel has great potential to be applied in flexible electronic wearable devices and sensor usages.To enhance the mechanical strength and conductivity, copolymer hydrogel is interpenetrated with CNT. In the past decade, several works have utilized this strategy to further optimize the performance of hydrogels.84,85 However, during the chemical treatment of CNT, its p-conjugation can be disrupted, affecting the performance of CNT on the electrical conductivity.86 A study reported by Li et al. provided a facile approach to tackle this challenge by interpenetrating single-walled carbon nanotubes (SWCNTs) into a pyrene-and-borate-modified P(DMA-co-APB-co-ABA) hydrogel composite, and such self-healable, electrical conductive hydrogels suggest a desirable future in e-skins.72 Nanosheets or nanocomposites are possible fillers for fabricating conductive hydrogels. Wang and co-authors intersected XLG nanosheets into SBMA-HEMA copolymers to fabricate a conductive nanocomposite hydrogel with self-healing ability, which can be applied to synthesize skin strain sensors.68 Notably, the XLT can adsorb zwitterions, generating reversible physical interaction among chains and improving mechanical properties.68
Besides adding CNT or nanosheet fillers, introducing conductive metal ions is also an excellent strategy to endow the system with high conductivity.65,69,71 Tan et al. reported a thermo-sensitive hydrogel by constructing the chemical and physical interaction between Al3+ and P(DMAPS-co-AA) copolymer.71 It reveals a promising application in electrocardiogram monitoring due to its enhanced conductivity and mechanical strength.71 Moreover, the metal ions can also form strong coordinate bonds with other polymer chains, improving the self-healing and mechanical properties of hydrogels.65,87 A typical example is the study reported by Das and co-authors, who proposed a poly(4-styrene sulfonate-co-methyl-uracil-imidazolium) chloride (PSS-MUI), followed by adding ferric ions as the second crosslinker into the gelatin system (Fig. 2a1 and a2).69 The as-prepared double-network hydrogel can be applied in wearable electronic devices (Fig. 2a3).69
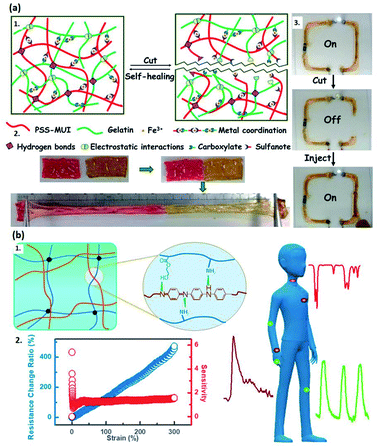 | ||
| Fig. 2 Conductive hydrogels fabricated via copolymerization approach. (a) Schematic representation of Fe3+-based conductive hydrogel. (a1) The mechanism of self-healing property. (a2) The stretchability of PSUGF-2 hydrogel strips after healing at room temperature for 120 min. (a3) The conductivity test for a flexible electric circuit assembled by PSUGF-2 hydrogel. (Adapted with permission from ref. 69. Copyright 2020, American Chemical Society). (b) Schematic illustration of (b1) the generated PANI/P(AAm-co-HEMA) hydrogels, (b2) the relationship between resistance change ratio and tensile strain, and (b3) the expected application in e-skins. (Adapted with permission from ref. 67. Copyright 2018, American Chemical Society). | ||
Another method to fabricate a conductive hydrogel with high mechanical properties via direct copolymerization is to build physical and chemical crosslinks between different polymer chains, combining the advantages of each polymer. Wang et al. developed a tough, conductive hydrogel by adding polyaniline (PANI) chains into the biocompatible P(AAm-co-HEMA) copolymer crosslink network, which is promising in flexible electronic devices by virtue of its high sensitivity and linearity in the presence of large strains (Fig. 2b1 and b2).67 PAM is also frequently introduced into the hydrogel systems to form crosslinks due to its superior flexibility and stretchability.88,89 Long et al. reported a strategy to fabricate a PDA-PAM hydrogel crosslinking network composed of self-polymerized PDA, AM-BIS copolymers, and tetramethylethylenediamine (TMEDA).73 The as-prepared hydrogels are further synthesized as an electrode in a sandwich configuration (PP-TENG).73 Similar methodology is also expected to be utilized to develop e-skins due to the excellent stretchability, self-repairing ability, and conductivity. To achieve more accurate control of the crosslinking network growth, Liu and co-authors utilized PEG-Gly as a template, where AM and AA monomers can be copolymerized, generating a PEG/PAMAA conductive hydrogel.70 Owing to the self-healing property and high sensitivity, it would have more hopeful prospects in flexible devices.70
Various works demonstrate the applications of conductive hydrogels prepared by direct copolymerization.90–92 Due to the superior stretchability of conductive hydrogels, they are typically chosen to assemble flexible strain sensors.93 For example, Liu et al. fabricated a wearable sweaty hydrogel strain sensor based on DNA-inspired block polymers, which exhibits desirable sensitivity to human motion, including head nodding, speaking, and joint bending.92 Notably, it is highly adhesive to sweat and water at different temperatures, indicating suitability to be applied in extreme environments.92 Solid-state supercapacitor is another example for e-skins, where hydrogels play a significant role in the conductive electrode owing to the high capacitive energy density and scalable processibility.94 Li et al. developed a flexible solid-state supercapacitor, which comprises polyaniline–polyvinyl alcohol hydrogel (PPH) as an electrode.95 It has superior specific capacitance, mechanical properties, and cyclic stability compared to other supercapacitors,95 leading it to be a promising candidate to integrate with wearable textiles and form flexible sensors for health monitoring.96 Apart from smart wearable sensors for human monitoring or motion detection, conductive hydrogels also show great potential in the robotic field to detect real-time actuations of robots.97 Yang and co-authors reported a high-performance rGO/poly(AMPS-co-AAm) nanocomposite hydrogel, which shows significant reversible and repeatable electro-response under cyclic applications of the electric field.90 Such hydrogels can be regarded as an actuator for cantilevers and grippers on soft robots.90
2.2 Grafting modification
Grafting is another critical strategy to synthesize conductive hydrogels on account of the porous structure in the hydrogel network, enabling the hydrogels to become more flexible and softer. Compared with traditional copolymerization approaches, grafting can further improve the crosslinking density and secondary interactions between polymer chains.74 For instance, Chen et al. fabricated a cellulose-based conductive hydrogel grafted with acrylonitrile and acrylamide copolymers, which can create secondary interactions between copolymer chains, improving the ultra-stretchability, toughness, and anti-freezing properties of the hydrogel.74 Such conductive hydrogels can be used to produce flexible strain sensors.74Grafting conductive oligomers, polymer chains, and nanocomposites is the common strategy to enhance the mechanical strength and conductivity of hydrogels.34,76,77,81 It is well established that aniline tetramer (AT) and PPy are desirable grafting agents owing to their high conductivity and biocompatibility.98,99 Zhao et al. developed a conductive injectable hydrogel by a thermal-gelling approach.76 Specifically, AT is grafted on poly(caprolactone)–poly(ethyleneglycol)–poly(caprolactone) copolymer and form amphiphilic AT-PCEC hydrogel with excellent biocompatibility,76 which is expected to be an excellent candidate for e-skins. Dong and co-authors also utilized AT to prepare a biodegradable injectable hydrogel, which consists of chitosan-graft-AT and PEG-DA copolymers and performs excellent self-repairing ability and antibacterial activity. This hydrogel is not only an ideal material to fabricate vehicles as cell delivery carriers,77 but also presents a wide range of possibilities for further development of e-skins due to its biocompatibility and conductivity.77 To further enhance the biocompatibility and self-healing ability, Wang et al. designed a gelatin-based injectable hydrogel by grafting conductive PPy on the MA-grafted gelatin hydrogel network and incorporated ferric ions into the system to enhance the conductivity.81 The hydrogel exhibits self-healing properties,81 making it a suitable material for e-skins. Besides grafting conductive polymer chains, Mandal and co-authors creatively utilized poly(acryloyl hydrazide)–silver nanocomposite reinforcements with different sizes, followed by grafting it into PAMAS hydrogel matrix. The constructed ionic linkages play a significant role in improving the mechanical strength of the hydrogel (Fig. 3a1).34 The as-prepared hydrogel shows high conductivity, toughness, and stretchability (Fig. 3a2), which can be applied in flexible device applications such as ECG monitoring (Fig. 3a3),34 raising the prospects in electronic skins.
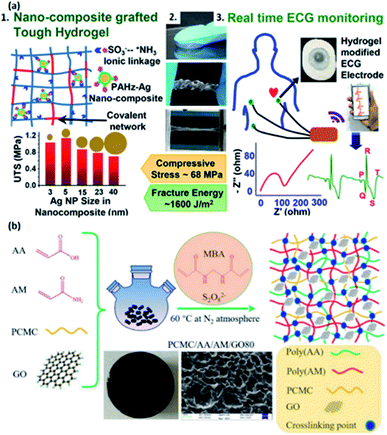 | ||
| Fig. 3 The representation of conductive hydrogels fabricated by grafting. (a) Nanocomposite-grafted PAMAS conductive hydrogel with (a1) the covalent network and ionic linkage, and (a2) the stretchability and toughness have improved. (a3) Modification on the ECG electrode for monitoring. (Adapted with permission from ref. 34. Copyright 2020, American Chemical Society). (b) Schematic representation for fabricating AA–AM-grafted cellulose hydrogel reinforced by the GO fillers. (Adapted with permission from ref. 78. Copyright 2019, Elsevier). | ||
During the grafting process, rGO or GO is typically incorporated in the hydrogel system to reinforce the grafted structure.35,75,78,79 Dai and co-authors filled GO into the acrylic acid–acrylamide (AA–AM) grafted cellulose hydrogel, which is highly responsive to pH, salt, and organic solvent, making it a good candidate in the drug delivery system.75 Their work might offer a new avenue for flexible electronic devices. Similarly, Liang et al. developed a P(QCSG-g-GM) copolymer, followed by adding GO fillers.79 The synthesized injectable hydrogel is anti-bacterial, conductive, and photothermal responsive.79 As a filler, GO endows the hydrogels with high conductivity and photothermal property,79 and it could be a desirable material for flexible electronic devices. On the other hand, rGO is also widely used to enhance the mechanical properties of hydrogels, and it exhibits better gel properties than GO.80 Liang et al. reported an HA-DA/rGO hydrogel by grafting hyaluronic acid onto dopamine, followed by incorporating rGO in the H2O2/HPR system.35 The self-healing, adhesive hydrogel has excellent injectability, satisfying the requirements in wound dressing,35 realizing a bright future in e-skins and wearable electronic devices. Likewise, Jafarigol et al. developed a carboxymethyl cellulose-based conductive hydrogel, which is grafted by AM–AC copolymer, and its swelling behavior is further investigated by adding rGO with different ratios (Fig. 3b).78 Such a hydrogel exhibits heat-resistant, electro-conductivity, and excellent mechanical properties, opening the door to new possibilities in flexible electronics and soft energy storage systems.78
Grafting modification is also a strategy that provides effective solutions to the applications of e-skins for conductive hydrogels.100,101 Strain sensors are widely fabricated using conductive hydrogels to optimize the sensitivity and stretchability of conventional sensors. Chen and co-authors developed a conductive cellulose hydrogel grafted by acrylonitrile and acrylamide copolymers. The hydrogel was further demonstrated as a suitable candidate for wearable strain sensors by virtue of its superior sensitivity and stability, which is desirable for human healthcare detections or motion monitoring.100 On the other hand, the improvement in crosslinking density by grafting modification enables the hydrogels to be more flexible without sacrificing their stability and mechanical strength,74 indicating a future perspective in the electrode, especially for supercapacitors or batteries. For instance, Huang et al. reported a PAM-grafted VSNPs conductive hydrogel film, which was utilized as an electrolyte in a supercapacitor, exhibiting great sensitivity and compressibility.74 Since few supercapacitors with super-compressive properties have been investigated,74 this research paves a new road on compressive energy devices and robotic systems.
3 Doping
As one of the most important strategies to modify conductive hydrogels on account of the three-dimensional tunable network, doping has captured a huge amount of interest already.37 The dopants play a significant role in enhancing the conductivity, mechanical strength, and stability of conductive hydrogels,27,31,33,44–48,102–110 and polymer dopants have gained more popularity than other dopants such as acids, additives, small molecules, or metal salts. The introduction of dopants to conductive hydrogels makes them easier to control for physical and chemical properties as well as morphology compared to the traditional conductive hydrogels.37 Hence, the doped conductive hydrogels are great candidates to synthesize e-skins.3.1 Polymer dopants
Polymer dopant is of great importance in modifying the desirable properties (conductivity, flexibility, biocompatibility, toughness, etc.) of hydrogels during the fabrication process. Representative polymer dopants such as PPy, PANI, and poly(3,4-ethylenedioxythiophene):polystyrenesulfonate (PEDOT:PSS) for conductive hydrogel synthesis are widely used because they overcome the poor conductivity and mechanical properties of traditional hydrogels and endows special features with promising utilization in energy storage systems, biomaterials, and sensors.31,47,48,107–110PPy, as a traditional dopant, can enhance the electrical conductivity and compatibility of hydrogels,107 expanding the possibilities of hydrogels for the development of e-skins or soft sensor applications. Bu et al. reported sodium alginate and carboxymethyl chitosan-based conductive hydrogel using PPy as a dopant, which exhibits biocompatibility and may achieve controllable conductivity and mechanical properties by adjusting the content of dopants.110 Therefore, this hydrogel is an ideal material for fabricating flexible electronics. Recently, Zhao et al. synthesized a tough and stretchable hydrogel based on chitosan network, PAA, and Fe3+, followed by doping PPy particles into the pre-hydrogel network, and thus the conductivity of the hydrogel can be enhanced (Fig. 4a1).107 The as-prepared hydrogel has excellent self-healing properties, which can be embedded into soft electronics or sensors (Fig. 4a2).107 By contrast, PPy can also be doped by other polymers to endow the hydrogel with other suitable properties. Han and co-authors developed a conductive hydrogel by incorporating dopant polydopamine into PPy fibers.47 The hydrophilicity of the polymer network enables it to further interweave with polyacrylamide to form PDA-PPy-PAM hydrogel.47 Notably, the hydrogel has a light-transmitting property, having wide applications such as transparent flexible electronics, biosignal detection, etc.47
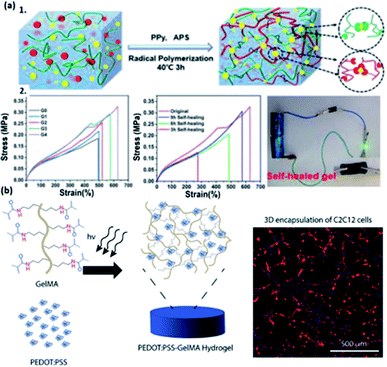 | ||
| Fig. 4 The illustration of representative conductive hydrogels with the modification of different types of polymer dopants. (a1) The mechanism of fabricating CS/PAA/PPy/Fe(III) hydrogels. (a2) Influence of self-healing time and Fe(III) on the mechanical properties of hydrogels and the conductive behavior of self-healed hydrogels. (Adapted with permission from ref. 107. Copyright 2021, American Chemical Society). (b) Fabrication of PEDOT:PSS-GelMA hydrogel and actin/DAPI image from C2C12 cells encapsulated in 0.1% PEDOT:PSS over 5 days of culture (scale bars = 500 μm). (Adapted with permission from ref. 48. Copyright 2018, American Chemical Society). | ||
Doping a polymer blend is also a strategy to synthesize conductive hydrogels such as PEDOT:PSS.109 As an example, Wu and co-authors fabricated a poly(N-acryloyl glycinamide-co-2-acrylamide-2-methylpropanesulfonic) conductive hydrogel, followed by doping with poly(3,4-ethylenedioxythiophene)–poly (styrenesulfonate) (PEDOT/PSS), featuring relatively high conductivity.109 The as-prepared hydrogel has excellent self-repairability, thermal processability, and stretchability, enabling the utilization in sensors and biomaterials.109 Taking advantage of the water-dispersive property of PEDOT:PSS as a dopant, Spencer and co-authors also developed a conductive hydrogel based on gelatin methacryloyl (GelMA) doped by PEDOT:PSS, exhibiting electrical-tunability, photo-sensitive capacity, and such hydrogels can be applied in biomaterial manufacturing,48 having a promising future in e-skins and flexible electronic devices (Fig. 4b).
The studies about polymer-doped conductive hydrogels reveal the possible applications of e-skins, including flexible strain sensors, bioelectronics, and electrodes.111,112 One interesting example is the hydrogel-based sensors and bioelectrodes developed by Han et al., which are fabricated by PDA-doped PPy-PAM conductive hydrogels.111 They can detect body motion without extra aids and show superior tissue adhesiveness.111 Besides, the as-prepared hydrogels have UV shielding ability,111 indicating a bright future in smart textiles. Similarly, Chen and co-authors fabricated a PANI/PSS hydrogel network and successfully assembled it into a strain sensor for accurate human movement detection, such as vocal vibration, wrist pulse, finger bending, etc.112 Such hydrogel-based strain sensors can have diverse utilizations in either healthcare or daily monitoring.
PANI is another important dopant to improve the conductivity of hydrogels with a simple fabrication approach.31 Doping PANI into different gel systems can achieve widespread applications of hydrogels, depending on the features of the system being doped. Das and co-authors adopted a hybrid PANI-doped conductive hydrogel, which is crosslinked by folic acid and embedded with AgNPS nanoparticles to improve the conductivity and photo-responsive properties, realizing a promising application in the energy storage field (Fig. 5a).108 Likewise, Chakraborty et al. successfully doped polyaniline into N-fluorenylmethoxycarbonyl diphenylalanine hydrogel, enhancing the conductivity and biocompatibility of the polymer, and the Fmoc-FF-PANI hydrogel has a promising future in sensing and biomedical fields.31 Such hydrogels could be used to synthesize flexible electronics. Moreover, PANI can also be combined with other strategies to prepare conductive hydrogels, such as doping by acid to enhance its conductivity, which will be discussed in the next section.
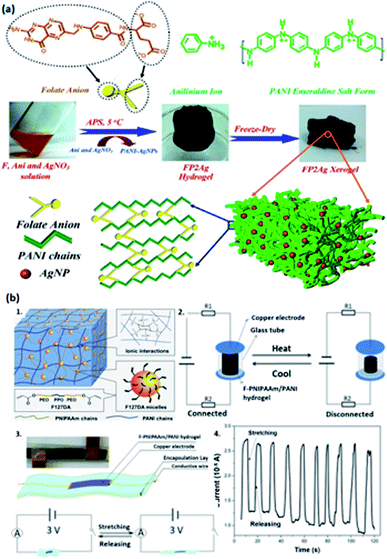 | ||
| Fig. 5 PANI-based conductive hydrogels. (a) Illustration of PANI-doped AgNPS/Fe3+ hydrogels and the crosslinking network. (Adapted with permission from ref. 108. Copyright 2018, American Chemical Society). (b) The representation of F-PNIPAAm/PANI hydrogels doped by PA. (b1) The network structure. (b2) The temperature alertor assembled by F-PNIPAAm/PANI hydrogels under cyclic heating and cooling. (b3) The configuration of a strain sensor based on-PNIPAAm/PANI hydrogels when being stretched and released under 3 V (Adapted with permission from ref. 33. Copyright 2018, American Chemical Society). (b4) The illustration of current response under 50% periodical tension strain of the strain sensor (Adapted with permission from ref. 33. Copyright 2018, American Chemical Society). | ||
3.2 Acid dopants
Acid dopants are used to endow the hydrogels with high conductivity by tuning the concentrations of the dopant so that different concentrations of charge carriers can be achieved.103 Consequently, doping acid into the conductive polymer is a facile methodology to adopt an enhancement on the conductivity of hydrogels through protonation to achieve equilibrium interactions between polymer chains to endow good mechanical capability of hydrogels,37 and such acid-doped conductive hydrogels have widespread applications such as flexible sensors, energy storage devices, and electrocatalysts.33,44–46,102,103Phytic acid (PA) is selected to dope a conductive hydrogel (typically PANI or PPy network) to enhance its electrical conductivity, which has a bright future in e-skins and flexible electronics.33,44–46 Wang et al. adopted a hydrogel with high stretchability due to its dually synergetic network, followed by incorporating electro-conductive PANI doped with PA into thermal-sensitive poly(N-isopropylacrylamide) (PNIPAAm), forming a crosslinked network (Fig. 5b1).33 The as-prepared F-PNIPAAm/PANI hydrogel has great potential in strain and temperature sensors (Fig. 5b2–b4).33 Similarly, Zhu and co-authors developed a hybrid conductive hydrogel by introducing PANI into polyion complex in the presence of PA as a dopant and crosslinker, which enhances the mechanical properties and conductivity of the hydrogel, and it is suitable for fabricating flexible electronics and strain sensors (Fig. 6a–c).44 Besides, PA can also be doped into PPy hydrogels; for example, Hu et al. doped phytic acid (PA) into conductive PPy, followed by coating with a carbon cloth to synthesize PA-PPy/CC hydrogel with superhydrophilic properties. Such hydrogels can be used as electrocatalysts for oxygen evolution reactions (OER).45
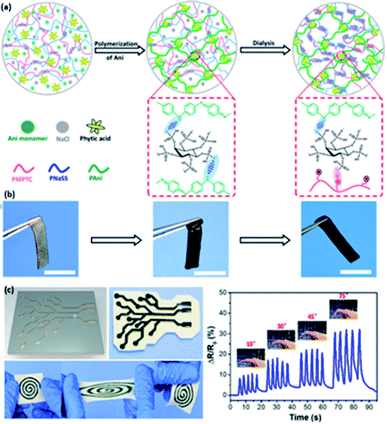 | ||
| Fig. 6 Illustration of conductive hydrogels based on PANI. (a) Fabrication approach for PIC/PAni hybrid gels doped by PA. (b) The images for PIC/PAni hybrid gels under various mechanical states during the fabrication. (c) The recoverability of the generated PIC/PAni hydrogel, and the behavior of the PIC/PAni hydrogel sensor in response to different magnitudes of repetitive finger bending. (Adapted with permission from ref. 44. Copyright 2018, American Chemical Society). | ||
Besides, other acid dopants are also useful in synthesizing conductive hydrogels, depending on the properties and features of each acid. Tannic acid is a suitable dopant for hydrogels in biomedical applications due to its high capability to improve both the mechanical strength and conductivity of hydrogels.113–116 For instance, Zhou and co-authors used tannic acid as a dopant and crosslinker to modify the PPy hydrogel backbone, which exhibits excellent conductivity.46 This biocompatible conductive polymer hydrogel can be applied in producing biomaterials.46 More recently, Zhou et al. synthesized a PAV/PANI hydrogel doped with TA, exhibiting suitable mechanical strength and conductivity by adjusting the concentrations of TA.102 Such hydrogels can be further applied to produce electrodes for pressure sensors owing to their excellent sensitivity and conductivity.102 On the other hand, since sulfuric acid contains high concentrations of protons, it could be used to control the ionic strength and water absorption ability of the hydrogel.103 Katzenberg et al. reported a facile method to synthesize polymer-electrolyte membranes, using conductive hydrogel as an electrolyte.103 Specifically, sulfuric acid is doped into a poly(acrylic acid)–poly(vinyl alcohol) network, increasing the conductivity of the system.103 Such homogenous, crosslinked structures are expected to be one of the most important alternative materials for e-skins.
Acid doping is a strategy that shows a powerful impact on the applications of electronic skins for conductive hydrogels.117–119 As for the Lithium Ion Battery (LIB) electrode, the conductive hydrogel framework is typically incorporated with various active materials and doped with acids to ensure the smooth transfer of electrons and ions.120,121 Shi and co-authors studied a 3D nanostructured PPy gel framework, which was employed as the binder and framework for the nanoparticle-based LIB electrode with superior capacity and conductivity.119 Furthermore, Fe3O4 nanoparticles were assembled into the gel framework to improve the electron and ion transport.119,121 Such an electrode is a promising candidate of anode material for the next-generation flexible LIBs.119 The second example is a piezoresistive sensor which was developed by Pan et al., which is composed of a PA-doped PPy hydrogel thin film with high elasticity and sensitivity, enabling the accurate detection of subtle variations of pressure, showing great potential in robotic and industrial detections.118 Besides, bioelectronics are widely developed using conductive hydrogels, where acid doping is typically used to enhance stability and conductivity. Zhang and co-authors reported an injectable, healable, and soft organic bioelectronics based on PEDOT:PSS conductive hydrogels, where 4-dodecylbenzenesulfonic acid (DBSA) as a dopant improved the ionic strength and protonation.117 Such bioelectronic can be potentially used as injectable conductors or further developed as organic electrochemical transistors (OECTs) for biomedical applications.117
3.3 Other dopants
Apart from polymers and acids, there are other types of dopants, such as additives, small molecule compounds, metal salts, etc., for the synthesis of hydrogels. They are used either to increase the conductivity and mechanical properties or achieve specific features of hydrogels,26,27,104–106,122 realizing the promising utilizations in e-skins.Additive dopants (rGO, CNT, clays, etc.) are widely used to enhance both the mechanical property and conductivity of hydrogels.26,105,106 Chen et al. synthesized a poly(2-acrylamido-2-methyl-1-propanesulfonic acid-co-acrylamide) hydrogel doped by rGO to increase the electronic conductivity of the system (Fig. 7a1 and a2).105 In their work, the hydrogel is further fabricated with a thermal-sensitive elastomer to form a core–shell segmental structure, having potential in flexible strain and temperature sensors and flexible electronic devices (Fig. 7a3).105 Similarly, CNT can also be doped into the hydrogel to endow conductivity. Roshanbinfar and co-authors reported a conductive biohybrid hydrogel by doping multiwall carbon nanotubes (MWCNT) into a pericardial matrix to generate PMCNT-hydrogel, which provides an appropriate environment for the pluripotent stem cell-derived cardiomyocytes growth.106 The conductivity and biocompatibility enable it to be a great candidate in e-skins. Interestingly, clays such as Laponite® may also be an excellent dopant with the conductive property. Tondera et al. prepared a conductive hydrogel with excellent stretchability and adhesivity, which can be used to fabricate materials in tissue engineering.26 After the doping by Laponite®, the poly(ethylene-3,4-diethoxy)-based hydrogel gained enhanced conductivity and mechanical properties.26 Such flexible, biocompatible hydrogels are a desirable material for fabricating e-skins.
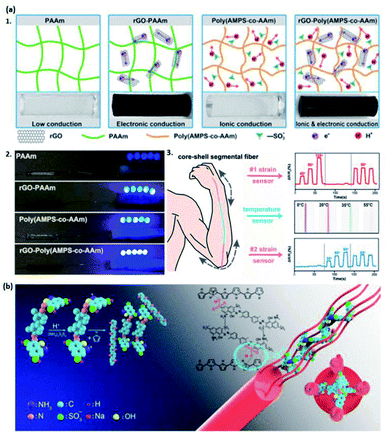 | ||
| Fig. 7 (a) The illustration of rGO-poly(AMPS-co-AAm) hydrogels with enhanced conductivity by (a1) designing ionic and electronic conductivities. (a2) The comparison of conductivity of the LED indicators for hydrogels containing different components. (a3) The potential applications of rGO-poly(AMPS-co-AAm) hydrogels in strain and temperature sensors. (Adapted with permission from ref. 105. Copyright 2018, American Chemical Society). (b) Schematic representation of the PPy-based hydrogel doped by small molecule compounds TB. (Adapted with permission from ref. 104. Copyright 2019, American Chemical Society). | ||
Besides, small molecule components are also reported as dopants in preparing conductive hydrogels.27,104 For example, Yang and co-authors developed a PPy-based conductive hydrogel and crosslinked by trypan blue (TB) as a dopant (Fig. 7b).104 By tuning the concentrations of TB, different electrochemical properties can be achieved, and this hydrogel is flexible and exhibits high biocompatibility,104 which is promising in designing e-skins or soft electronics. Also, Ding et al. reported a conductive hydrogel network by polymerizing PANI nanostructure in the presence of heparin as a dopant, which is crosslinked by methacrylate.27 It exhibits excellent conductivity and biocompatibility. Such hydrogels can be applied in biomaterials in tissue engineering,27 and are expected to be used in flexible electronics.
Another possible dopant for conductive hydrogels is metal salt, which promotes the ionic conductivity of the system. Recently, Yin et al. adopted a polypyrrole sodium alginate hydrogel doped with ferric chloride and sodium dodecylbenzene sulfonate, respectively.122 Specifically, the former hydrogel exhibits better stability and electrochemical properties, but both of them are ideal materials to fabricate e-skins and flexible electronics.122
Several studies have demonstrated the diverse applications for these materials, which depend on the types of dopants and the functionalities the hydrogel provides.123,124 A typical case is the biosensor based on conductive hydrogels with superior flexibility and sensitivity, which are the most favored for either food detection such as food and water contaminant tests or healthcare monitoring to accurately assess the diagnosis of patients.125 For instance, Ma et al. designed a wireless sensor for food spoilage detection, which was assembled by p-toluene sulfonate hexahydrate (PTS)-doped PANI hydrogels.124 The material is highly sensitive to amines and meets the requirements for food detection since PTS is allowed to be used as a food additive based on the United States Food and Drug Administration (FDA) regulations.124 On the other hand, a nitrogen-doped graphene hydrogel reported by Chen and co-authors illustrated an effective solution in supercapacitors since it showed ultrafast discharge/charge rate and higher power density, indicating promising utilization in vehicle, lifts, or flexible robot systems.123
4 Self-assembly
Compared with polymerization, conductive hydrogels prepared by self-assembly can typically achieve weaker interactions such as p–p stacking, electrostatic interactions, van der Waals forces, hydrogen bonds, and hydrophobic interactions.126–137 Accordingly, better functionalities, mechanical properties, and interfacial affinity can be achieved for self-assembled hydrogels, improving the biocompatibility of synthetic polymers.38 Moreover, self-assembly plays a vital role in generating multifunctional conductive hydrogels in comparison with the copolymerization and doping strategies because no reactions are involved during this process. In this way, self-assembled conductive hydrogels have attracted widespread attention, and they are promising candidates in e-skins.4.1 π–π stacking
Induced by the strong interaction, π–π stacking is one of the most important contributors for the self-assembly of conductive hydrogels. To endow these hydrogels with high conductivity, some great candidates could be graphene oxide (GO) or conductive molecules and polymers, and all of them contain conjugated p bonds, generating high strength for hydrogels.126–131 Such kinds of conductive hydrogels may potentially be used in flexible electronics and e-skins.In general, the graphene-based component is selected as a raw material to synthesize conductive hydrogels due to its excellent conductivity and improved mechanical properties of stacking by the conjugated bonds.126,128,130,131 Introducing a one-pot hydrothermal reaction is a typical way to form graphene-based conductive hydrogels through the self-assembly process.126,128,130,131 Xu and co-authors (2010) first demonstrated a facile one-step hydrothermal approach to construct a graphene-based hydrogel, featuring electrical conductivity, mechanical properties, thermal stability, and biocompatibility (Fig. 8a).131 Owing to the π–π interaction as the main driving force, the two-dimensional GO can self-assemble into the 3D hydrogel network.131 The as-prepared hydrogel is expected to be applied in flexible electronics because of its biocompatibility and conductivity. Likewise, Sheng and co-authors fabricated self-assembled chemical-reduced GO sheets via π–π stacking with the promotion of sodium ascorbate, forming three-dimensional graphene hydrogel by hydrothermal reaction, and it is an ideal material in supercapacitor manufacturing.130 Moreover, graphene oxide can be incorporated with DNA chains to provide biocompatibility for the hydrogels so that they have potential in biochemical applications. Xu et al. (2010) successfully adopted a multifunctional hydrogel, having excellent mechanical strength, self-repairing ability, and can be used to prepare graphene-based biomaterials,126 flexible electronics devices, and e-skins. Furthermore, the DNA chains and GO nanosheets are driven by π–π interactions through self-assembly, binding them together into a 3D network.126 Besides, if GO nanosheets are combined with other particles, the mechanical properties of the hydrogel could be further enhanced. Zhang et al. developed a 3D nanocomposite conductive hydrogel based on self-assembled graphene nanosheets and TiO2 nanoparticles via a one-pot hydrothermal reaction.128 It is noteworthy that the π–π stacking existing between GO nanosheets enables the formation of self-assembled hydrogels, which can be applied in fabricating energy storage devices.128
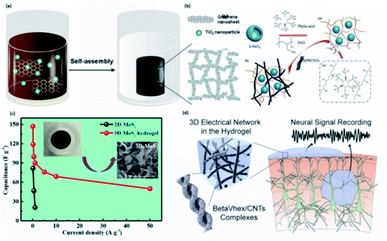 | ||
| Fig. 8 The self-assembled conductive hydrogels are driven by four main types of secondary interactions. (a) Schematic illustration of graphene nanosheets driven by π–π stacking through self-assembly. (Adapted with permission from ref. 111). (Copyright 2013, American Chemical Society). (b) The mechanism of supramolecular self-assembly of PAH wrapped SnO2 anode and the addition of MWCNT to achieve electron transfer. (Adapted with permission from ref. 133. Copyright 2016, Springer). (c) The illustration of MoS2-based hydrogel shows higher capacitance than 2D-MoS2. (Adapted with permission from ref. 137. Copyright 2019, American Chemical Society). (d) The representation of self-assembled betaVhex nanofibers and betaVhex/CNTs complexes to form a hydrogel system and its utilization in neural signal monitoring. (Adapted with permission from ref. 148. Copyright 2020, American Chemical Society). | ||
Other materials such as conductive molecules and polymer blending may also be driven by the π–π interaction.127,129 Components with low molecular weight combined with metal cations is typically used for supramolecular assembly of conductive hydrogels,138,139 and π–π stacking as one major driving force for conjugated molecules, endowing excellent mechanical properties and self-healing capability.129 Hu et al. developed a conductive hydrogel by incorporating adenosine 5′-monophosphate (AMP) and manganese ions (Mn2+) through self-assembly to synthesize AMP-Mn hydrogel, followed by crosslinking with PVA.129 The self-assembly is due to the existence of π–π stacking between nucleobases, and the as-prepared hydrogel has wide applications in energy storage,129 enabling it to be a desirable candidate for e-skins. On the other hand, polymer blending is regarded as a viable source for fabricating host–guest self-assembly of conductive hydrogels.127 Xu et al. (2019) successfully synthesized a conductive hydrogel via host–guest self-assembly of poly-β-cyclodextrin (Pβ-CD) and PEDOT blended with adamantyl-modified sulfated alginate (S-Alg-Ad) through π–π stacking interactions.127 Such hydrogels have high mechanical properties, self-healing ability, and injectability127 and may be utilized in soft electronics or e-skins.
The studies about conductive hydrogels driven by π–π stacking reveal the effective solutions of supercapacitors, endowing them with superior flexibility, stretchability, and high surface area for electrochemical reactions.120,140,141 Xu and co-authors have successfully developed a graphene/Ni(OH)2 composite hydrogel, which was further assembled into a high-performance solid-state supercapacitor.140 After that, Xu et al. reported another flexible supercapacitor fabricated by a 3D graphene hydrogel film, formed via π–π stacking.141 Such supercapacitors can achieve desirable gravimetric specific capacitance, cyclic stability, and excellent mechanical strength, indicating a bright future for flexible energy storage devices.141 Besides the role as electronic skins for healthcare or biomedical applications, hydrogel-based flexible supercapacitors may also have wide utilization in electronic paper, energy driven devices, robotic systems, and roll-up displays, paving a path on designing smart electronics with more functionalities.142
4.2 Electrostatic interaction and van der Waals forces
Electrostatic interactions and van der Waals forces are two types of noncovalent interactions with similar strengths between polymer chains.143 However, van der Waals forces are generally the weak interactions between neutral molecules or polymers, while the electrostatic interaction involves the components with ionic groups.144 Both of them play an important role in driving the formation of conductive hydrogels. In the typical strategy, the self-assembled networks are incorporated with mechanically strong additives such as nanosheets or GOs to endow the hydrogels with robustness. Thus, they are the desirable materials in flexible electronic devices and biomaterial synthesis.132–134Electrostatic interactions are frequently employed to design conductive hydrogels via self-assembly, which refers to two groups of molecules with opposite charges attracted to each other,145 forming a noncovalent interaction and contributing to the driven force to generate conductive hydrogels through self-assembly. Typically, the self-assembly process takes place between small natural molecules or polymer chains.132,133 For example, Patel et al. reported a hydrogel-based nanocomposite fibrous film fabricated by conductive graphene and two kinds of biocompatible polysaccharides (chitosan and gellan gum).132 The existence of electrostatic interactions between positively-charged chitosan and negatively-charged gellan gum contributes to the main driving force of self-assembly.132 The addition of graphene enables excellent wettability, conductivity, and mechanical properties of the hydrogel, and it has great potential in biomaterial applications.132 In their work, since the film has a unidirectional hierarchy, promoting the myoblasts to align correctly, and therefore, it is expected to design cell-instructive biomaterials.132 Also, Shi and co-authors mainly utilized the electrostatic interaction between the phytic acid (gelator) and poly(allylamine hydrochloride) (PAH) chains, followed by incorporation with SnO2, which is expected to have the excellent capacity by self-assembly process (Fig. 8b).133 Then, the prepared hydrogel is combined with MWCNT to improve the conductivity of the whole system. Specifically, there is a synergistic effect between PAH and MWCNTs and improving the conductivity and self-healing ability of the hydrogel is benefited from the existence of electrostatic effects and ionic interactions.133 Therefore, such hydrogels have a promising application in manufacturing anode for lithium-ion batteries,133 which could be further designed to synthesize e-skins or flexible electronics.
Introducing van der Waals force is also a promising strategy to synthesize conductive hydrogels through the self-assembly process. With the functionalization of specific groups on the materials, the self-assembly process can achieve different properties. For instance, Liu et al. developed a conductive hydrogel using a surface charge control strategy, and it is based on the self-assembly of three-dimensional molybdenum disulfide (MoS2) nanosheets formed through van der Waals interaction (Fig. 8c).137 Unlike the traditional MoS2 material, this MoS2-based hydrogel exhibits higher capacitance, having great potential in fabricating supercapacitors,137 approaching a promising application in e-skins.
The existence of electrostatic interactions or van der Waals forces between polymer chains endows conductive hydrogels with excellent mechanical properties, indicating a promising future in flexible strain sensors.146,147 Wang et al. synthesized a PVA hydrogel reinforced with zwitterionic PSBMA chains via electrostatic and dipole–dipole interactions.146 The as-prepared hydrogels were utilized as a flexible strain sensor for pulse detection, showing superior accuracy and sensitivity to the subtle physiological signals.146 Similarly, a flexible and wearable strain sensor was also developed by Xia et al., which is composed of polyacrylamide (PAAm)/chitosan (CS) hybrid hydrogels, and the conductive c-MWCNTs were fixed on the network through electrostatic interactions.147 The physiological signals such as bending, movement of the waist, neck, and elbows can also be monitored once the hydrogel-based strain sensor is attached to human skin.147
4.3 Self-assembly based on other stronger interactions
Among all the self-assembly approaches through noncovalent interactions to prepare conductive hydrogels, hydrogen bonding and hydrophobic interactions are two types of forces, which are much stronger than others. Normally, the conductivity is achieved by incorporating conductive polymers or CNTs through self-assembly. Meanwhile, the mechanical properties of hydrogels can also be enhanced, enabling them to be applied in flexible electronics.36,134Hydrogen bonding can be utilized to design conductive hydrogels via the self-assembly process, and due to its higher bond energy, the hydrogels are robust and have excellent properties. For example, Nam et al. reported a peptide-based conductive hydrogel with biocompatibility and biostability by self-assembling betaVhex and peptide via hydrogen bond interactions to synthesize betaVhex, followed by incorporating conductive CNT (Fig. 8d).148 Then, the prepared betaVhex/CNTs hydrogel is used to form a soft neural interface, which can magnify the signals and prevent the signal from being dissipated.148 Such materials can be further applied to make e-skins.
Similarly, hydrophobic interaction is also used to drive the formation of the hydrogel by self-assembly. In fact, the hydrophobic interactions are generally stronger than that of hydrogen bonds, leading to relatively high mechanical properties and fast self-repairing ability of hydrogels,135 and the self-assembly performance depend on plenty of factors such as temperature, hydrophobic functional groups, and the shape of materials.149 In most cases, the hydrophobic interaction takes place between nanoparticles, driving the formation of self-assembled networks.135,136 Xu et al. developed an electroconductive hydrogel based on self-assembling PEDOT:PSS and PEG-peptide together, which is mainly driven by hydrophobic interactions between PEDOT nanoparticles, and p–p stacking among PSS and PEDOT, attributing to the self-healing ability of the hydrogel, and it can be applied in adopting cell cultures.135 Recently, Gao and co-authors successfully developed a DNA-based hydrogel, which was incorporated by conductive CNTs and AuNPs nanoparticles through self-assembly.136 In their work, the DNA chains are wrapped around the CNT walls via strong hydrophobic interactions, promoting the assembly of the hydrogel, which exhibits excellent conductivity and mechanical properties, approaching a more desirable application in flexible electronic devices.136
Hydrogen bond and hydrophobic powered conductive hydrogels demonstrate a strong impact in the applications of electronic skins, especially for flexible strain sensors for monitoring devices in the biomedical field.150,151 Liu et al. prepared a strain sensor based on GelMA-TA-carbon hydrogel, which was driven by hydrogen bonding with superior mechanical properties, flexibility, and adhesiveness.150 The as-prepared material was further demonstrated as a wound-healing material, indicating a new innovation in the biomedical area.150 Besides, Ye and co-authors reported a multi-functional organohydrogel sensor for human motion detection under either large or subtle changes of pressure.151 Notably, upon both compressive and tensile deformation, the sensor can maintain its sensitivity, stability, and durability,151 suggesting extensive utilization in healthcare, injectable materials, or seawater desalination.
5 Perspective and discussion
Conducive hydrogel is of utmost importance for e-skins by virtue of its flexibility, stretchability, self-healing properties, sensitivity, and adhesiveness,66–73,86,152 which have made it a promising alternative for traditional inorganic conductive materials.153 Thus, its fabrication strategies continue to be a great impetus to achieve incremental performances of conductive hydrogels.As one of the most popular approaches, copolymerization can not only control the crosslink density and ion contents in the hydrogel network by directly tuning the ratios among different monomers,66 but is also useful in modifying the special functional groups on the hydrogel backbones and forming covalent crosslinking and physical interactions among chains through grafting.72,74 However, it is time-consuming and requires strict controls for preparation,154 and lacks mechanical and conductive stability when conductive hydrogels are prepared.23
Compared to copolymerization, doping can also be employed to modify the mechanical and conductive properties by controlling the amount of dopants.107 But what really matters is, based on this chemical approach, the functions of the hydrogels are much easier to modulate with high accuracy, and there is a possibility for dopants to exhibit reversible properties in the hydrogel structures, endowing high sensitivity and multifunctional responsiveness for the hydrogels.37 Moreover, the performance of self-assembled conductive hydrogels can be further enhanced using dopants, which help to tailor the morphology of the self-assembled hydrogels due to the unique functional groups in dopants,37 forming conductive hydrogels for various functions. Hence, doping can be regarded as a complementary strategy for copolymerization and self-assembly in fabricating conductive hydrogels, raising the prospects in the applications of flexible electronics.
As mentioned above, self-assembly is also a critical approach to fabricate supramolecular hydrogels, endowing the hydrogels with self-healing ability, which plays a significant role for electronic skins.129 Owing to the various categories of driving forces, such as p–p stacking,126–131 hydrophobic interaction,36,135,136 van der Waals forces,137 hydrogen bonds134 and electrostatic interactions,132,133 self-assembly can be adopted into different kinds of hydrogel synthesis, conferring a superior degree of self-healing capacity in the materials.
Based on these three fundamental strategies for the rational design of conductive polymers, particularly for hydrogels, we should pour much more attention on developing combined strategies such as polymerization-doping or self-assembly-doping, realizing superior mechanical strength conductivity and functionality simultaneously via different optimization mechanisms (Fig. 9).
In summary, this review discusses the recent advances of conductive hydrogels and proposes a theoretical framework to synthesize conductive hydrogels, which can be mainly classified into copolymerization, doping, and self-assembly, and their advantages and disadvantages are also discussed critically. Copolymerization and self-assembly methods can generate tunable and self-healable conductive hydrogels, respectively, while doping can usually be utilized to improve the stability and functionalities of hydrogels. Furthermore, the potential applications for conductive hydrogels in e-skins are also highlighted.
In the future, further efforts are still required to figure out appropriate combinations of possible strategies in terms of copolymerization, doping, and self-assembly. These chemical methodologies can be applied to design a new class of chemical tools in fabricating conductive hydrogels so its performance in the utilization of e-skins and flexible electronic devices can be further optimized. Thus, it is expected that in the future, these three approaches will still dominate in synthesizing conductive hydrogels with improved mechanical, conductive properties and multifunctionalities, providing a breakthrough in the usages of e-skins.
Author contributions
C. J. Li conceived the whole manuscript and organized the materials. The author has approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge Dr Han Zhang and Dr Dimitrios Papageorgiou at Queen Mary University of London for their patient and professional guidance about hydrogels, which provides the inspiration for the review. The author also thanks Miss Jingyi Shi, Ziyang Li, Peishan Li, Zekun Liu, Xinyu Zhao for their support and suggestions on the coursework. We gratefully acknowledge the constructive suggestions provided by Dr. Wei HUANG from the Chinese University of Hong Kong.Notes and references
- Z. Wang, Y. Cong and J. Fu, J. Mater. Chem. B, 2020, 8, 3437–3459 RSC
.
- T.-H. Han, H. Kim, S.-J. Kwon and T.-W. Lee, Mater. Sci. Eng., R, 2017, 118, 1–43 CrossRef
.
- Z. Zhang, Z. Chen, Y. Wang and Y. Zhao, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 18310 CrossRef CAS PubMed
.
- S. Zhang, S. Li, Z. Xia and K. Cai, J. Mater. Chem. B, 2020, 8, 852–862 RSC
.
- S. R. Forrest, Nature, 2004, 428, 911–918 CrossRef CAS PubMed
.
- Z. Wang, Y. Cong and J. Fu, J. Mater. Chem. B, 2020, 8, 3437–3459 RSC
.
- D.-H. Kim, J.-H. Ahn, W. M. Choi, H.-S. Kim, T.-H. Kim, J. Song, Y. Y. Huang, Z. Liu, C. Lu and J. A. Rogers, Science, 2008, 320, 507 CrossRef CAS PubMed
.
- G.-T. Hwang, D. Im, S. E. Lee, J. Lee, M. Koo, S. Y. Park, S. Kim, K. Yang, S. J. Kim, K. Lee and K. J. Lee, ACS Nano, 2013, 7, 4545–4553 CrossRef CAS PubMed
.
- K. Liu, S. Wei, L. Song, H. Liu and T. Wang, J. Agric. Food Chem., 2020, 68, 7269–7280 CrossRef CAS PubMed
.
- X. Yang, X. Zhang, Q. Guan and X. Zhang, J. Mater. Chem. C, 2021, 9, 2815–2822 RSC
.
- J. Zhang, Z. Lei, S. Luo, Y. Jin, L. Qiu and W. Zhang, ACS Appl. Nano Mater., 2020, 3, 4845–4850 CrossRef CAS
.
- X. Liu, Y.-Z. Long, L. Liao, X. Duan and Z. Fan, ACS Nano, 2012, 6, 1888–1900 CrossRef CAS PubMed
.
- W. K. Chee, H. N. Lim, Z. Zainal, N. M. Huang, I. Harrison and Y. Andou, J. Phys. Chem. C, 2016, 120, 4153–4172 CrossRef CAS
.
- H.-P. Phan, Y. Zhong, T.-K. Nguyen, Y. Park, T. Dinh, E. Song, R. K. Vadivelu, M. K. Masud, J. Li, M. J. A. Shiddiky, D. Dao, Y. Yamauchi, J. A. Rogers and N.-T. Nguyen, ACS Nano, 2019, 13, 11572–11581 CrossRef CAS PubMed
.
- D. Shahrjerdi and S. W. Bedell, Nano Lett., 2013, 13, 315–320 CrossRef CAS PubMed
.
- K.-J. Moon, T.-I. Lee, J.-H. Choi, J. Jeon, Y. H. Kang, J. P. Kar, J. H. Kang, I. Yun and J.-M. Myoung, ACS Nano, 2011, 5, 159–164 CrossRef CAS PubMed
.
- X. Liu, Y.-Z. Long, L. Liao, X. Duan and Z. Fan, ACS Nano, 2012, 6, 1888–1900 CrossRef CAS PubMed
.
- J. P. Rojas, G. A. Torres Sevilla, M. T. Ghoneim, S. B. Inayat, S. M. Ahmed, A. M. Hussain and M. M. Hussain, ACS Nano, 2014, 8, 1468–1474 CrossRef CAS PubMed
.
- K. Teng, Q. An, Y. Chen, Y. Zhang and Y. Zhao, ACS Biomater. Sci. Eng., 2021, 7, 1302–1337 CrossRef CAS PubMed
.
- Y. Lee, J. Park, A. Choe, S. Cho, J. Kim and H. Ko, Adv. Funct. Mater., 2020, 30, 1904523 CrossRef CAS
.
- Y. Cao, Y. J. Tan, S. Li, W. W. Lee, H. Guo, Y. Cai, C. Wang and B. Tee, Nat. Electron., 2019, 2, 75 CrossRef
.
- J. Stejskal, Chem. Pap., 2017, 71, 269–291 CrossRef CAS
.
- F. Fu, J. Wang, H. Zeng and J. Yu, ACS Mater. Lett., 2020, 2, 1287–1301 CrossRef CAS
.
- T.-H. Le, Y. Kim and H. Yoon, Polymers, 2017, 9(4), 150 CrossRef PubMed
.
- K. Liu, S. Wei, L. Song, H. Liu and T. Wang, J. Agric. Food Chem., 2020, 68, 7269–7280 CrossRef CAS PubMed
.
- C. Tondera, T. F. Akbar, A. K. Thomas, W. Lin, C. Werner, V. Busskamp, Y. Zhang and I. R. Minev, Small, 2019, 15, 1901406 CrossRef PubMed
.
- H. Ding, M. Zhong, Y. J. Kim, P. Pholpabu, A. Balasubramanian, C. M. Hui, H. He, H. Yang, K. Matyjaszewski and C. J. Bettinger, ACS Nano, 2014, 8, 4348–4357 CrossRef CAS PubMed
.
- Y. Zhao, C. Shi, X. Yang, B. Shen, Y. Sun, Y. Chen, X. Xu, H. Sun, K. Yu, B. Yang and Q. Lin, ACS Nano, 2016, 10, 5856–5863 CrossRef CAS PubMed
.
- M. Choi, J. W. Choi, S. Kim, S. Nizamoglu, S. K. Hahn and S. H. Yun, Nat. Photonics, 2013, 7, 987–994 CrossRef CAS PubMed
.
- I. Jeon, J. Cui, W. R. K. Illeperuma, J. Aizenberg and J. J. Vlassak, Adv. Mater., 2016, 28, 4678–4683 CrossRef CAS PubMed
.
- P. Chakraborty, T. Guterman, N. Adadi, M. Yadid, T. Brosh, L. Adler-Abramovich, T. Dvir and E. Gazit, ACS Nano, 2019, 13, 163–175 CrossRef CAS PubMed
.
- Y.-J. Liu, W. Cao, M.-G. Ma and P. Wan, ACS Appl. Mater. Interfaces, 2017, 9 (30), 25559–25570 CrossRef PubMed
.
- Z. Wang, H. Zhou, W. Chen, Q. Li, B. Yan, X. Jin, A. Ma, H. Liu and W. Zhao, ACS Appl. Mater. Interfaces, 2018, 10, 14045–14054 CrossRef CAS PubMed
.
- S. Mandal, A. Seth, V. Yadav, S. Kumari, M. Kumar and U. Ojha, ACS Appl. Polym. Mater., 2020, 2, 618–625 CrossRef CAS
.
- Y. Liang, X. Zhao, T. Hu, B. Chen, Z. Yin, P. X. Ma and B. Guo, Small, 2019, 15, 1900046 CrossRef PubMed
.
- J. Nam, H.-K. Lim, N. H. Kim, J. K. Park, E. S. Kang, Y.-T. Kim, C. Heo, O.-S. Lee, S.-G. Kim, W. S. Yun, M. Suh and Y. H. Kim, ACS Nano, 2020, 14, 664–675 CrossRef CAS PubMed
.
- Z. Ma, W. Shi, K. Yan, L. Pan and G. Yu, Chem.
Sci., 2019, 10, 6232–6244 RSC
.
- M. Halperin-Sternfeld, M. Ghosh and L. Adler-Abramovich, in Supramolecular Chemistry of Biomimetic Systems, ed. J. Li, Springer Singapore, Singapore, 2017, pp. 9–35 Search PubMed
.
- M. W. Handlogten, J. Wang and S. Ahuja, Biotechnol. Bioeng., 2020, 117, 1329–1336 CrossRef CAS PubMed
.
- F. Alam and K. Balani, Adhesion force of staphylococcus aureus on various biomaterial surfaces, J. Mech. Behav. Biomed. Mater., 2017, 65, 872–880 CrossRef CAS PubMed
.
- F. Alam and K. Balani, J. Mech. Behav. Biomed. Mater., 2017, 65, 872–880 CrossRef CAS PubMed
.
- Y. Yu, H. Yuk, G. A. Parada, Y. Wu, X. Liu, C. S. Nabzdyk, K. Youcef-Toumi, J. Zang and X. Zhao, Adv. Mater., 2019, 31, 1807101 CrossRef PubMed
.
- I. Jeon, J. Cui, W. R. K. Illeperuma, J. Aizenberg and J. J. Vlassak, Adv. Mater., 2016, 28, 4678–4683 CrossRef CAS PubMed
.
- F. Zhu, J. Lin, Z. L. Wu, S. Qu, J. Yin, J. Qian and Q. Zheng, ACS Appl. Mater. Interfaces, 2018, 10, 13685–13692 CrossRef CAS PubMed
.
- Q. Hu, G. Li, X. Liu, B. Zhu, X. Chai, Q. Zhang, J. Liu and C. He, Angew. Chem., Int. Ed., 2019, 58, 4318–4322 CrossRef CAS PubMed
.
- L. Zhou, L. Fan, X. Yi, Z. Zhou, C. Liu, R. Fu, C. Dai, Z. Wang, X. Chen, P. Yu, D. Chen, G. Tan, Q. Wang and C. Ning, ACS Nano, 2018, 12, 10957–10967 CrossRef CAS PubMed
.
- L. Han, L. Yan, M. Wang, K. Wang, L. Fang, J. Zhou, J. Fang, F. Ren and X. Lu, Chem. Mater., 2018, 30, 5561–5572 CrossRef CAS
.
- A. R. Spencer, A. Primbetova, A. N. Koppes, R. A. Koppes, H. Fenniri and N. Annabi, ACS Biomater. Sci. Eng., 2018, 4, 1558–1567 CAS
.
- B. Liu, P. Soares, C. Checkles, Y. Zhao and G. Yu, Nano Lett., 2013, 13, 3414–3419 CrossRef CAS PubMed
.
- F. Wang, J. Tseng, Z. Liu, P. Zhang, G. Wang, G. Chen, W. Wu, M. Yu, Y. Wu and X. Feng, Adv. Mater., 2020, 32, 2000287 CrossRef CAS PubMed
.
- Z. Wang, H. Zhou, J. Lai, B. Yan, H. Liu, X. Jin, A. Ma, G. Zhang, W. Zhao and W. Chen, J. Mater. Chem. C, 2018, 6, 9200–9207 CAS
.
- V. P. Chuang, J. Y. Cheng, T. A. Savas and C. A. Ross, Nano Lett., 2006, 6, 2332–2337 CrossRef CAS PubMed
.
- L. He, Q. Xiao, Y. Zhao, J. Li, S. Reddy, X. Shi, X. Su, K. Chiu and S. Ramakrishna, ACS Appl. Mater. Interfaces, 2020, 12, 53150–53163 CrossRef CAS PubMed
.
- Y. Hu, P. Shen, N. Zeng, L. Wang, D. Yan, L. Cui, K. Yang and C. Zhai, ACS Appl. Mater. Interfaces, 2020, 12, 42285–42293 CrossRef CAS PubMed
.
- Y. Xu, M. Cui, P. A. Patsis, M. Günther, X. Yang, K. Eckert and Y. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 7715–7724 CrossRef CAS PubMed
.
- Y. Xu, X. Yang, A. K. Thomas, P. A. Patsis, T. Kurth, M. Kräter, K. Eckert, M. Bornhäuser and Y. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 14418–14425 CrossRef CAS PubMed
.
- M. R. Kesama, S. R. Dugasani, Y. J. Cha, J. Son, B. Gnapareddy, S. Yoo, D. K. Yoon and S. H. Park, Nanotechnology, 2019, 30, 245704 CrossRef CAS PubMed
.
- J. Nam, H.-K. Lim, N. Kim, J. Park, E. Kang, Y. Kim, C. Heo, O.-S. Lee, S.-G. Kim, W. Yun, M. Suh and Y. Kim, ACS Nano, 2020, 14, 664–675 CrossRef CAS PubMed
.
- Encyclopedia of Cancer, ed. M. Schwab, Springer Berlin Heidelberg, Berlin, Heidelberg, 2009, pp. 958–958 Search PubMed
.
- A. Patel, Y. Xue, R. Hartley, V. Sant, J. R. Eles, X. T. Cui, D. B. Stolz and S. Sant, Biotechnol. Bioeng., 2018, 115, 2654–2667 CrossRef CAS PubMed
.
- F. Poncin and G. Legeay, J. Biomater. Sci., Polym. Ed., 2003, 14, 1005–1028 CrossRef PubMed
.
- K. G. Neoh, R. Wang and E. T. Kang, in Biomaterials and Medical Device – Associated Infections, ed. L. Barnes and I. R. Cooper, Woodhead Publishing, Oxford, 2015, pp. 133–161 Search PubMed
.
- X. Peng, F. Zhao, Y. Peng, J. Li and Q. Zeng, Soft Matter, 2020, 16, 54–63 RSC
.
- R. Balint, N. J. Cassidy and S. H. Cartmell, Acta Biomater., 2014, 10, 2341–2353 CrossRef CAS PubMed
.
- F. Fu, J. Wang, H. Zeng and J. Yu, ACS Mater. Lett., 2020, 2, 1287–1301 CrossRef CAS
.
- L. Jiang, C. Gentile, A. Lauto, C. Cui, Y. Song, T. Romeo, S. M. Silva, O. Tang, P. Sharma, G. Figtree, J. J. Gooding and D. Mawad, ACS Appl. Mater. Interfaces, 2017, 9, 44124–44133 CrossRef CAS PubMed
.
- Z. Wang, J. Chen, Y. Cong, H. Zhang, T. Xu, L. Nie and J. Fu, Chem. Mater., 2018, 30, 8062–8069 CrossRef CAS
.
- L. Wang, G. Gao, Y. Zhou, T. Xu, J. Chen, R. Wang, R. Zhang and J. Fu, ACS Appl. Mater. Interfaces, 2019, 11, 3506–3515 CrossRef CAS PubMed
.
- S. Das, P. Martin, G. Vasilyev, R. Nandi, N. Amdursky and E. Zussman, Macromolecules, 2020, 53, 11130–11141 CrossRef CAS
.
- S. Liu, O. Oderinde, I. Hussain, F. Yao and G. Fu, Polymer, 2018, 144, 111–120 CrossRef CAS
.
- Y. Tan, Y. Zhang, Y. Zhang, J. Zheng, H. Wu, Y. Chen, S. Xu, J. Yang, C. Liu and Y. Zhang, Chem. Mater., 2020, 32, 7670–7678 CrossRef CAS
.
- X. Li, X. Huang, H. Mutlu, S. Malik and P. Theato, Soft Matter, 2020, 16, 10969–10976 RSC
.
- Y. Long, Y. Chen, Y. Liu, G. Chen, W. Guo, X. Kang, X. Pu, W. Hu and Z. L. Wang, Nanoscale, 2020, 12, 12753–12759 RSC
.
- D. Chen, X. Zhao, X. Wei, J. Zhang, D. Wang, H. Lu and P. Jia, ACS Appl. Mater. Interfaces, 2020, 12, 53247–53256 CrossRef CAS PubMed
.
- H. Dai, Y. Zhang, L. Ma, H. Zhang and H. Huang, Carbohydr. Polym., 2019, 215, 366–376 CrossRef CAS PubMed
.
- X. Zhao, B. Guo and P. X. Ma, J. Mater. Chem. B, 2015, 3, 8459–8468 RSC
.
- R. Dong, X. Zhao, B. Guo and P. X. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 17138–17150 CrossRef CAS PubMed
.
- E. Jafarigol, M. B. Salehi and H. R. Mortaheb, Chem. Eng. Res. Des., 2020, 162, 74–84 CrossRef CAS
.
- Y. Liang, B. Chen, M. Li, J. He, Z. Yin and B. Guo, Biomacromolecules, 2020, 21, 1841–1852 CrossRef CAS PubMed
.
- Mandeep, A. Gulati and R. Kakkar, Chem. Eng. Res. Des., 2020, 153, 21–36 CrossRef CAS
.
- S. Wang, J. Lei, X. Yi, L. Yuan, L. Ge, D. Li and C. Mu, ACS Appl. Polym. Mater., 2020, 2, 3016–3023 CrossRef CAS
.
- C. Cui, Q. Fu, L. Meng, S. Hao, R. Dai and J. Yang, ACS Appl. Bio Mater., 2021, 4, 85–121 CrossRef CAS
.
- X. Han, G. Xiao, Y. Wang, X. Chen, G. Duan, Y. Wu, X. Gong and H. Wang, J. Mater. Chem. A, 2020, 8, 23059–23095 RSC
.
- X.-W. Liu, Y.-X. Huang, X.-F. Sun, G.-P. Sheng, F. Zhao, S.-G. Wang and H.-Q. Yu, ACS Appl. Mater. Interfaces, 2014, 6, 8158–8164 CrossRef CAS PubMed
.
- A. A. Adewunmi, S. Ismail and A. S. Sultan, J. Inorg. Organomet. Polym. Mater., 2016, 26, 717–737 CrossRef CAS
.
- K. Balasubramanian and M. Burghard, J. Mater. Chem., 2008, 18, 3071–3083 RSC
.
- Z. Zhang, Z. Gao, Y. Wang, L. Guo, C. Yin, X. Zhang, J. Hao, G. Zhang and L. Chen, Macromolecules, 2019, 52, 2531–2541 CrossRef CAS
.
- B. Lv, X. Bu, Y. Da, P. Duan, H. Wang, J. Ren, B. Lyu, D. Gao and J. Ma, Polymer, 2020, 210, 123021 CrossRef CAS
.
- O. Zhanadilov, A. Mentbayeva, Z. Beisbayeva, M. Amze and Z. Bakenov, Mater. Today: Proc., 2021 DOI:10.1016/j.matpr.2020.11.989
.
- C. Yang, Z. Liu, C. Chen, K. Shi, L. Zhang, X.-J. Ju, W. Wang, R. Xie and L.-Y. Chu, ACS Appl. Mater. Interfaces, 2017, 9, 15758–15767 CrossRef CAS PubMed
.
- Y. Shi, L. J. Pan, B. Liu, Y. Wang, Y. Cui, Z. Bao and G. Yu, J. Mater. Chem. A, 2014, 2, 6086–6091 RSC
.
- X. Liu, Q. Zhang and G. Gao, Chem. Eng. J., 2020, 394, 124898 CrossRef CAS
.
- L. Tang, S. Wu, J. Qu, L. Gong and J. Tang, Materials, 2020, 13(18), 3947 CrossRef CAS PubMed
.
- H. Zhang, M. Yue, T. Wang, J. Wang, X. Wu and S. Yang, New J. Chem., 2021, 45, 4647–4657 RSC
.
- W. Li, F. Gao, X. Wang, N. Zhang and M. Ma, Angew. Chem., Int. Ed., 2016, 55, 9196–9201 CrossRef CAS PubMed
.
- S. Banerjee, B. De, P. Sinha, J. Cherusseri and K. K. Kar, in Handbook of Nanocomposite Supercapacitor Materials I: Characteristics, ed. K. K. Kar, Springer International Publishing, Cham, 2020, pp. 341–350 Search PubMed
.
- L.-Y. Hsiao, L. Jing, K. Li, H. Yang, Y. Li and P.-Y. Chen, Carbon, 2020, 161, 784–793 CrossRef CAS
.
- J. Yang, C.-R. Han, J.-F. Duan, F. Xu and R.-C. Sun, Nanoscale, 2013, 5, 10858–10863 RSC
.
- Y. Wu, B. Guo and P. X. Ma, ACS Macro Lett., 2014, 3, 1145–1150 CrossRef CAS
.
- D. Chen, X. Zhao, X. Wei, J. Zhang, D. Wang, H. Lu and P. Jia, ACS Appl. Mater. Interfaces, 2020, 12, 53247–53256 CrossRef CAS PubMed
.
- Y. Huang, M. Zhong, F. Shi, X. Liu, Z. Tang, Y. Wang, Y. Huang, H. Hou, X. Xie and C. Zhi, Angew. Chem., Int. Ed., 2017, 56, 9141–9145 CrossRef CAS PubMed
.
- H. Zhou, M. Wang, X. Jin, H. Liu, J. Lai, H. Du, W. Chen and A. Ma, ACS Appl. Mater. Interfaces, 2021, 13, 1441–1451 CrossRef CAS PubMed
.
- A. Katzenberg, C. Muñoz Davila, B. Chen, T. Siboonruang and M. A. Modestino, ACS Appl. Polym. Mater., 2020, 2, 2046–2054 CrossRef CAS
.
- C. Yang, P. Zhang, A. Nautiyal, S. Li, N. Liu, J. Yin, K. Deng and X. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 4258–4267 CrossRef CAS PubMed
.
- J. Chen, H. Wen, G. Zhang, F. Lei, Q. Feng, Y. Liu, X. Cao and H. Dong, ACS Appl. Mater. Interfaces, 2020, 12, 7565–7574 CrossRef CAS PubMed
.
- K. Roshanbinfar, Z. Mohammadi, A. Sheikh-Mahdi Mesgar, M. M. Dehghan, O. P. Oommen, J. Hilborn and F. B. Engel, Biomater. Sci., 2019, 7, 3906–3917 RSC
.
- L. Zhao, X. Li, Y. Li, X. Wang, W. Yang and J. Ren, Biomacromolecules, 2021, 22(3), 1273–1281 CrossRef CAS PubMed
.
- S. Das, P. Chakraborty, S. Mondal, A. Shit and A. K. Nandi, ACS Appl. Mater. Interfaces, 2016, 8, 28055–28067 CrossRef CAS PubMed
.
- Q. Wu, J. Wei, B. Xu, X. Liu, H. Wang, W. Wang, Q. Wang and W. Liu, Sci. Rep., 2017, 7, 41566 CrossRef PubMed
.
- Y. Bu, H.-X. Xu, X. Li, W.-J. Xu, Y. Yin, H. Dai, X. Wang, Z.-J. Huang and P.-H. Xu, RSC Adv., 2018, 8, 10806–10817 RSC
.
- L. Han, L. Yan, M. Wang, K. Wang, L. Fang, J. Zhou, J. Fang, F. Ren and X. Lu, Chem. Mater., 2018, 30(16), 5561–5572 CrossRef CAS
.
- J. Chen, Q. Peng, T. Thundat and H. Zeng, Chem. Mater., 2019, 31(12), 4553–4563 CrossRef CAS
.
- H. Ejima, J. Richardson, K. Liang, J. Best, M.G. van KoeverdenSuch, J. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed
.
- S. Quideau, D. Deffieux and C. Douat-Casassus, Angew. Chem., 2011, 50, 586–621 CrossRef CAS PubMed
.
- M. Shin, H.-A. Lee, M. Lee, Y. Shin, J.-J. Song, S.-W. Kang, D.-H. Nam, E. J. Jeon, M. Cho, M. Do, S. Park, M. S. Lee, J.-H. Jang, S.-W. Cho, K.-S. Kim and H. Lee, Nat. Biomed. Eng., 2018, 2, 304–317 CrossRef CAS PubMed
.
- F. Behboodi-Sadabad, H. Zhang, V. Trouillet, A. Welle, N. Plumeré and P. A. Levkin, Adv. Funct. Mater., 2017, 27, 1700127 CrossRef
.
- S. Zhang, Y. Chen, H. Liu, Z. Wang, H. Ling, C. Wang, J. Ni, B. Çelebi-Saltik, X. Wang, X. Meng, H.-J. Kim, A. Baidya, S. Ahadian, N. Ashammakhi, M. R. Dokmeci, J. Travas-Sejdic and A. Khademhosseini, Adv. Mater., 2020, 32, 1904752 CrossRef CAS PubMed
.
- L. Pan, A. Chortos, G. Yu, Y. Wang, S. Isaacson, R. Allen, Y. Shi, R. Dauskardt and Z. Bao, Nat. Commun., 2014, 5, 3002 CrossRef PubMed
.
- Y. Shi, J. Zhang, A. M. Bruck, Y. Zhang, J. Li, E. A. Stach, K. J. Takeuchi, A. C. Marschilok, E. S. Takeuchi and G. Yu, Adv. Mater., 2017, 29, 1603922 CrossRef PubMed
.
- W. Zhang, P. Feng, J. Chen, Z. Sun and B. Zhao, Prog. Polym. Sci., 2019, 88, 220–240 CrossRef CAS
.
- D. C. Bock, K. C. Kirshenbaum, J. Wang, W. Zhang, F. Wang, J. Wang, A. C. Marschilok, K. J. Takeuchi and E. S. Takeuchi, ACS Appl. Mater. Interfaces, 2015, 7, 13457–13466 CrossRef CAS PubMed
.
- J. Yin, Q. Liu, J. Zhou, L. Zhang, Q. Zhang, R. Rao, S. Liu and T. Jiao, RSC Adv., 2020, 10, 10546–10551 RSC
.
- P. Chen, J.-J. Yang, S.-S. Li, Z. Wang, T.-Y. Xiao, Y.-H. Qian and S.-H. Yu, Nano Energy, 2013, 2, 249–256 CrossRef CAS
.
- Z. Ma, P. Chen, W. Cheng, K. Yan, L. J. Pan, Y. Shi and G. Yu, Nano Lett., 2018, 18(7), 4570–4575 CrossRef CAS PubMed
.
- A. Haleem, M. Javaid, R. P. Singh, R. Suman and S. Rab, Sensors International, 2021, 2, 100100 CrossRef
.
- Y. Xu, Q. Wu, Y. Sun, H. Bai and G. Shi, ACS Nano, 2010, 4, 7358–7362 CrossRef CAS PubMed
.
- Y. Xu, M. Cui, P. A. Patsis, M. Günther, X. Yang, K. Eckert and Y. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 7715–7724 CrossRef CAS
.
- Z. Zhang, F. Xiao, Y. Guo, S. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 2227–2233 CrossRef CAS PubMed
.
- Y. Hu, P. Shen, N. Zeng, L. Wang, D. Yan, L. Cui, K. Yang and C. Zhai, ACS Appl. Mater. Interfaces, 2020, 12, 42285–42293 CrossRef CAS PubMed
.
- K. Sheng, Y. Xu, C. Li and G. Shi, New Carbon Mater., 2011, 26, 9–15 CrossRef CAS
.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS PubMed
.
- A. Patel, Y. Xue, R. Hartley, V. Sant, J. R. Eles, X. T. Cui, D. B. Stolz and S. Sant, Biotechnol. Bioeng., 2018, 115, 2654–2667 CrossRef CAS PubMed
.
- Y. Shi, D. Ma, W. Wang, L. Zhang and X. Xu, J. Mater. Sci., 2017, 52, 3545–3555 CrossRef CAS
.
- J. Nam, H.-K. Lim, N. H. Kim, J. K. Park, E. S. Kang, Y.-T. Kim, C. Heo, O.-S. Lee, S.-G. Kim, W. S. Yun, M. Suh and Y. H. Kim, ACS Nano, 2020, 14, 664–675 CrossRef CAS PubMed
.
- Y. Xu, X. Yang, A. K. Thomas, P. A. Patsis, T. Kurth, M. Kräter, K. Eckert, M. Bornhäuser and Y. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 14418–14425 CrossRef CAS PubMed
.
- M. Gao, A. Krissanaprasit, A. Miles, L. C. Hsiao and T. H. LaBean, J. Appl. Sci., 2021, 11 Search PubMed
.
- C. Liu, X. Zhao, S. Wang, Y. Zhang, W. Ge, J. Li, J. Cao, J. Tao and X. Yang, ACS Appl. Energy Mater., 2019, 2, 4458–4463 CrossRef CAS
.
- M.-O. M. Piepenbrock, G. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110(4), 1960–2004 CrossRef CAS PubMed
.
- M. Krogsgaard, V. Nue and H. Birkedal, Chemistry, 2016, 22, 844–857 CrossRef CAS PubMed
.
- Y. Xu, X. Huang, Z. Lin, X. Zhong, Y. Huang and X. Duan, Nano Res., 2013, 6, 65–76 CrossRef CAS
.
- Yuxi Xu, Xiaoqing Huang, Zhaoyang Lin, Xing Zhong, Yu Huang and Xiangfeng Duan, Nano Res., 2013, 6, 65–76 CrossRef CAS
.
- X. Liang, G. Long, C. Fu, M. Pang, Y. Xi, J. Li, W. Han, G. Wei and Y. Ji, Chem. Eng. J., 2018, 345, 186–195 CrossRef CAS
.
- B. Shi, Z. Wang and H. Wen, J. Mol. Liq., 2017, 241, 486–488 CrossRef CAS
.
- T. Tsuchiya, in Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth, ed. W. M. White, Springer International Publishing, Cham, 2018, pp. 1473–1474 Search PubMed
.
- Encyclopedia of Cancer, ed. M. Schwab, Springer Berlin Heidelberg, Berlin, Heidelberg, 2009, pp. 958–958 Search PubMed
.
- Z. Wang, J. Chen, L. Wang, G. Gao, Y. Zhou, R. Wang, T. Xu, J. Yin and J. Fu, J. Mater. Chem. B, 2019, 7, 24–29 RSC
.
- S. Xia, S. Song, F. Jia and G. Gao, J. Mater. Chem. B, 2019, 7, 4638–4648 RSC
.
- J. Nam, H.-K. Lim, N. H. Kim, J. K. Park, E. S. Kang, Y.-T. Kim, C. Heo, O.-S. Lee, S.-G. Kim, W. S. Yun, M. Suh and Y. H. Kim, ACS Nano, 2020, 14, 664–675 CrossRef CAS PubMed
.
- F. Xiao, Z. Chen, Z. Wei and L. Tian, Adv. Sci., 2020, 7, 2001048 CrossRef CAS PubMed
.
- B. Liu, Y. Wang, Y. Miao, X. Zhang, Z. Fan, G. Singh, X. Zhang, K. Xu, B. Li, Z. Hu and M. Xing, Biomaterials, 2018, 171, 83–96 CrossRef CAS PubMed
.
- Y. Ye, Y. Zhang, Y. Chen, X. Han and F. Jiang, Adv. Funct. Mater., 2020, 30, 2003430 CrossRef CAS
.
- Q. Rong, W. Lei and M. Liu, Chem.–Eur. J., 2018, 24, 16930–16943 CrossRef CAS PubMed
.
- Z. Wang, H. Zhou, W. Chen, Q. Li, B. Yan, X. Jin, A. Ma, H. Liu and W. Zhao, ACS Appl. Mater. Interfaces, 2018, 10, 14045–14054 CrossRef CAS PubMed
.
- H. Ji, X. Song, H. Cheng, L. Luo, J. Huang, C. He, J. Yin, W. Zhao, L. Qiu and C. Zhao, ACS Appl. Mater. Interfaces, 2020, 12, 31079–31089 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2021 |