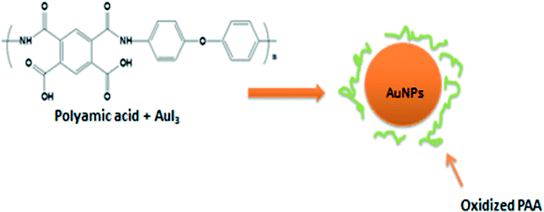
Synthesis and catalytic, antimicrobial and cytotoxicity evaluation of gold and silver nanoparticles using biodegradable, Π-conjugated polyamic acid†
Victor M.
Kariuki
a,
Idris
Yazgan
a,
Ali
Akgul
b,
Andrzej
Kowal
c,
Magdalena
Parlinska
d and
Omowunmi A.
Sadik
*a
aDepartment of Chemistry Center for Advanced Sensors & Environmental Systems (CASE), State University of New York, P.O. Box 6000, Binghamton, NY 13902, USA. E-mail: osadik@binghamton.edu
bDepartment of Basic Sciences, College of Veterinary Medicine, Mississippi State University, USA
cInstitute of Non-ferrous Metals Division in Poznan, Central Laboratory of Batteries and Cells, Poznan, Poland
dFacility for Electron Microscopy & Sample Preparation, University of Rzeszow, Rzeszow, Poland
First published on 18th August 2015
Abstract
We hereby report a rapid, simple, and one pot synthesis of silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) using conductive, electroactive and biodegradable poly(amic)acid (PAA) polymer as both the reductant and the stabilizer. The synthesized AgNPs and AuNPs were characterized using transmission electron microscopy (TEM), high-resolution TEM (HRTEM), energy-dispersive spectroscopy (EDS), X-ray diffraction (XRD) and ultraviolet-visible (UV-vis) spectroscopy. UV-vis spectra exhibit major peaks at 440 and 535 nm for AgNPs and AuNPs, respectively. The XRD patterns revealed four diffraction peaks at 38.12°, 44.07°, 64.27°, and 77.22° that can be indexed to the (111), (200), (220), and (311) planes of face-centered cubic (fcc) silver crystallites, respectively. The size of the crystallites along the [111] direction was estimated to be 4.2 ± 0.5 nm, which is in agreement with the TEM result. The effect of temperature on the formation of AgNPs in the presence of PAA was investigated and found to be significant at 100 °C, resulting in a silver–polyamic acid nanocomposite without altering the fcc crystal pattern. The prepared AuNPs and AgNPs were found to exhibit catalytic activity towards 4-nitrophenol and methylene blue with a rate constant of 5.2 × 10−3 s−1 and 1.09 × 10−2 s−1, respectively. Finally, the synthesized AgNPs exhibit excellent antibacterial activity against Gram negative (E. coli DH5 Alpha, E. coli 25922, Aeromonas hydrophila and Pseudomonas aeruginosa) and Gram positive (Listeria monocytogenes strains F2365 and HCC7 and S. epidermidis) bacteria in addition to modest cytotoxicity against non-cancerous immortalized IEC-6 and cancerous Caco-2 cell lines.
Nano impactThis study presents for the first time the use of environment-friendly poly(amic) acid (PAA) to synthesize polymer-supported gold (AuNPs) and silver nanoparticles (AgNPs). PAA shows great potential as both reductant and stabilizer of AuNPs and AgNPs, with the resultant nanoparticles retaining their catalytic and antimicrobial activity. This environment-friendly approach utilizing the tunable properties of PAA opens a new frontier for nanomaterials that can be used for catalysis, filtration, sensors and other applications. |
1. Introduction
Gold and silver nanoparticles have attracted a considerable surge of research interest due to their various inherent advantages such as the ease of synthesis, high chemical stability and size-dependent optical, electronic, and catalytic properties. This has led to diverse applications including chemical/biological sensing, surface-enhanced Raman scattering, (nano) electronics, catalysis, drug delivery, sensors, and antibacterial serum.1–7 However, due to their specific surface energy, they tend to agglomerate easily, resulting in the loss of nano-particular characteristics. One strategy to prevent aggregation of the nanoparticles is to utilize appropriate stabilizers. An excellent stabilizer must not mask the properties of the nanoparticles. It has been shown that the presence of a densely packed capping layer on a metal nanoparticle surface may compromise the catalytic activity as surface active sites become inaccessible and a compact layer introduces steric hindrance to the inward diffusion of reactants to the nanoparticle surface and outward diffusion of reaction products.8–10Moreover, there has been a recent surge of interest in the synthesis and applications of intrinsically conducting polymers (ICPs) with incorporated metal nanoparticles.11,12 Several intrinsically conducting polymers such as polyaniline13,14 and polypyrrole15 have been used as reductants and stabilizers of gold and silver nanoparticles. However, the application of polyaniline16 is limited due to its low processability, insolubility in a wide range of solvents and non-biodegradability. Notably, metal nanoparticles supported by PANI alone are not stable, forming aggregates in solution even after being left to stand for a few minutes.17 Similarly, polypyrrole is insoluble in a wide range of solvents.
Intrinsically conducting polymer molecules containing active groups and a considerable level of steric hindrance are believed to be more advantageous as stabilizers of metal nanoparticles. PAA, an electroactive,18,19 conductive18,20 and biodegradable21,22 polymer, contains numerous amounts of amide and carboxyl functional groups. Also, PAA has high cation-complexing abilities,19,23,24 implying that polymeric metal complexes can easily be generated by in situ reactions between PAA and labile salts of noble metals. Unlike other ICPs such as polyaniline and polypyrrole, PAA has excellent biodegradable characteristics and hence will not introduce any environmental toxicity. In this work, we used π-conjugated, biodegradable PAA as support and reducing agent to prepare AuNPs and AgNPs. The resulting nanoparticles exhibit excellent dispersibility and stability. Most importantly, the resulting AuNPs demonstrate apparent catalytic activity for the reduction of 4-nitrophenol to 4-aminophenol while the AgNPs demonstrate excellent antimicrobial and catalytic activity.
2. Materials and methods
2.1 Reagents
Silver nitrate (AgNO3), gold(III)iodide (AuI3), pyromellitic dianhydride (PMDA), 4,4′-oxydianiline, dimethylformamide (DMF), 4-nitrophenol, methylene blue, dimethyl acetamide (DMAC), Muller Hinton Broth, Alamar Blue and sodium borohydride (NaBH4) were purchased from Sigma Aldrich, St. Louis, MO, USA. All reagents were of analytical grade and used without further purification.2.2 Synthesis of poly(amic) acid (PAA)
210.25 mg of 4,4′-oxydianiline was added into a vial followed by 3.5 to 4 mL of DMF dispensed using a syringe. This mixture was stirred until complete dissolution of ODA was observed. This was followed by the addition of 229.02 mg of PMDA and 1 mL of DMAC. The vial was then covered with either a box container or aluminum foil after capping and stirred for 12 hours. The resulting PAA was a yellow viscous solution.2.3 Preparation of gold nanoparticles (AuNPs)
2.88 mg of AuI3 was dissolved in 5 mL of DMF and PAA solution was added. The reaction mixture was observed at room temperature with a solution of AuI3 in DMF used as control. The setup was monitored over time and UV-vis spectroscopy was used to characterize the formation of AuNPs.2.4 Preparation of silver nanoparticles (AgNPs)
The following setups were used for the synthesis of silver nanoparticles:(A) 5 mg of AgNO3 + 5 mL of DMF + PAA (0.21 M) was monitored at room temperature for 5 days.
(B) 5 mg of AgNO3 + 5 mL of DMF+ PAA (0.21 M) was monitored at 70 °C for 150 minutes
(C) 5 mg of AgNO3 + 5 mL of DMF+ PAA (0.21 M) was monitored at 100 °C for 200 minutes
2.5 Characterization
UV-vis spectra of the synthesized nanoparticles were recorded at different aging times using a Hewlett Packard 8453 UV-vis spectrophotometer from 200 nm to 700 nm. TEM measurements were conducted using a JEOL 2100F transmission electron microscope operating at an acceleration voltage of 200 kV. The TEM samples were prepared by dropping a few drops of the final products on a SiO2-coated copper grid (TedPella) and drying under a dryer for 5 minutes. XRD patterns were obtained using a Bruker D8 Discover XRD system operating at 40 kV and 40 mA. The diffraction patterns were recorded in θ–2θ symmetry scanning mode within the range of 30° to 90°. Test samples were prepared by dropping about 10 mL of silver or gold colloids on quartz plates and allowing them to dry.2.6 Reduction of 4-nitrophenol catalyzed by PAA-synthesized AuNPs
A typical mixture for the reduction consists of a 300 μL PAA-Au solution (5 × 10−5 M in water), 850 μL of 4-nitrophenol (0.2 mM aqueous solution) and 500 μL of freshly prepared NaBH4 solution (25 mM aqueous solution) in a 5 mL glass vial. The mixture was quickly transferred to a cuvette for UV-vis absorption measurements. Nanopure water was used as the reference. All experiments proceeded under ambient conditions.2.7 Reduction of methylene blue catalyzed by PAA-synthesized AgNPs
The catalytic properties of PAA-synthesized AgNPs were evaluated using a standard methylene blue reduction reaction. Two reaction setups were employed and monitoring was achieved using a UV-visible spectrophotometer in the range of 400–800 nm. In the first setup, 1 mL of methylene blue (1 × 10−4 M) was mixed with an excess of 32 mM NaBH4 and the mixture was monitored for 30 minutes. In the second setup, 1 mL of methylene blue was mixed with an excess of 32 mM NaBH4 in the presence of 400 μL of PAA-synthesized AgNPs (5 × 10−5 M) and the reaction was monitored for 3 minutes.2.8 Evaluation of antimicrobial, antifungal and cytotoxic characteristics of PAA-stabilized AgNPs
The antibacterial and antifungal activity of PAA-synthesized silver nanoparticles were tested to examine how they alter the growth dynamics of Gram positive and Gram negative bacteria. The Gram negative bacteria examined include Escherichia coli ATCC® 25922™, E. coli DH5 Alpha, Aeromonas hydrophila, and Pseudomonas aeruginosa. The Gram positive bacteria tested were Staphylococcus epidermidis ATCC® 12228™ and Listeria monocytogenes strains F2365 and HCC7. Antifungal activity was tested using the addition of Trichaptum biforme into a freshly prepared 10 μg mL−1 silver-nanoparticle-containing Mueller Hinton Broth. E. coli 25922 and S. epidermidis were grown in Mueller Hinton Agar, while virulent type strain L. monocytogenes were grown in a rich medium such as brain heart infusion; lysogeny broth (LB), a nutritionally rich agar medium, was used for the maintenance of the tested E. coli DH5 Alpha, Aeromonas hydrophila and Pseudomonas aeruginosa.The effect of PAA-synthesized silver nanoparticles on microbial growth was tested using two approaches: (i) bacteria were incubated in the liquid phase (broth) and (ii) solid phase, respectively. The concentrations of AgNPs used for antimicrobial activity varied for the type of bacteria within the range of 1–20 μg mL−1. Turbidity test was performed by dissolving the resulting colonies in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 phosphate-buffered saline (PBS) buffer
50 phosphate-buffered saline (PBS) buffer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Muller Hinton Broth. The absorbance of the Mueller Hinton Broth mixture was used to calculate the bacterial concentration using a standard curve.
Muller Hinton Broth. The absorbance of the Mueller Hinton Broth mixture was used to calculate the bacterial concentration using a standard curve.
The cytotoxicity of silver nanoparticles was tested on passage 31–35 IEC-6 [DMEM medium with 0.1 μg mL−1 bovine insulin, 10% FBS and a 1% penicillin and streptomycin mixture] and passage 85–90 Caco-2 cell lines [DMEM with 10% FBS and a 1% penicillin and streptomycin mixture]. While 10 μg mL−1 AgNPs was used for Caco-2 cells, 5, 10, 15 and 30 μg mL−1 nanoparticles were used for IEC-6 cells. Toxicity was evaluated with PrestoBlue® dye and comparisons of the final confluence of control and silver nanoparticle treated wells were carried out. The details are described in the ESI† (Fig. S10).
3. Results and discussion
3.1 Reaction mechanism for the formation of nanoparticles
Polymers such as polypyrrole and polyaniline are readily transformed to their respective oxidized forms by in situ reduction of metal ions such as Pd2+ and Au3+.25,26 In this study, we used PAA which possess electron-donating properties23 to reduce Au3+ to Au0 and Ag+ to Ag0. The oxidized polymer also protects and stabilizes the AuNPs and AgNPs in accordance with the following reactions:| Au3+ + 3PAA0(s) → Au0 + 3PAA+ | (1) |
| Ag+ + PAA0(s) → Ag0 + PAA+ | (2) |
The use of PAA results in the effective reduction of gold and silver salts while also stabilizing the nanoparticles, thus providing excellent robustness against agglomeration (Fig. 1).27 PAAs possess lone pairs of electrons on the nitrogen species capable of adsorbing onto the nanoparticles' surface and stabilizing them via steric hindrance (Fig. 1).28
3.2 Optical characterization
It is well known that nanoparticles exhibit a distinct color in aqueous solution due to the excitation of surface plasmon vibrations (SPRs).29Fig. S1A† shows the pictograph for the mixture of gold and PAA with the color changing from yellow to purple. A similar setup without the PAA remained yellow throughout the experiment. These results indicate the formation of AuNPs. Similarly, the formation of AgNPs was accompanied by color changes over time, with the color changing from pale yellow of the PAA matrix to bright yellow, to light reddish brown and finally to deep reddish brown (Fig. S1B and C and S2†). This was observed in all the setups at different temperatures (room temperature, 70 °C and 100 °C).
The solutions presented a favorable homogeneity without precipitation, indicating that the nanoparticles were stable and well dispersed in the PAA medium. The origin of the color changes is derived from the different states of the SPR of the nanoparticles at their characteristic wavelengths which were registered by the UV-vis absorption spectra.30
3.3 UV-vis characterization
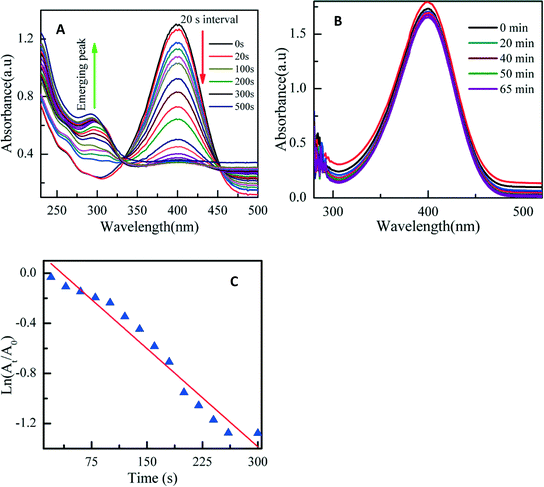
As evident in Fig. 2A, the AuNP absorption band appeared at 535 nm. It can be concluded that the band is due to the SPR of the gold nanoparticles at 510–525 nm in an aqueous system.31 It is evident that the absorption band of PAA-protected gold nanoparticles was shifted compared with the calculated value. Gold nanoparticles of 5–50 nm show a sharp absorption band in the 520–530 nm region.32 The size deduced from UV-vis data agrees with TEM and XRD characterization. In the case of AgNPs, reduction occurred slowly at room temperature and up to 5 days was needed to observe any noticeable color change (Fig. S1C†). At 70 °C, it took 15 minutes (Fig. S2†) to record any color change, while only 5 minutes was needed to observe any visible color change at 100 °C (Fig. S1B†). This trend implies that higher temperature speeds the formation of nanoparticles.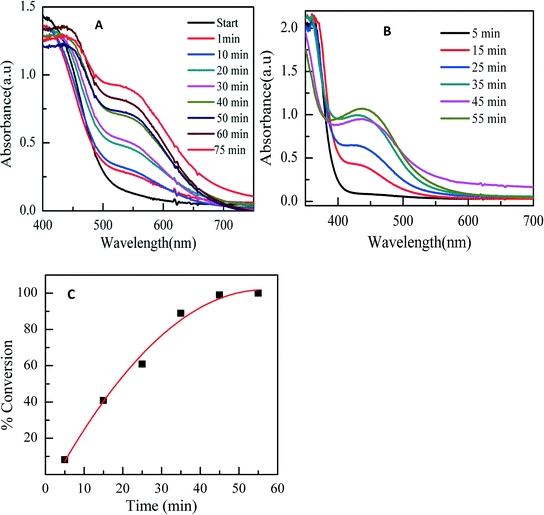 | ||
| Fig. 2 Time evolution of UV-vis absorption spectra of reaction mixture. (A) AuNPs at room temperature. (B) AgNPs at 100 °C. (C) Progress of the reaction for AgNP formation at 100 °C. | ||
The colloids formed were stable over 2 months with no precipitation. The characteristic brown color of silver solutions provided a noticeable spectroscopic signature to indicate their formation.33 The color of the silver colloid is attributed to the collective oscillation of free conduction electrons induced by an interacting electromagnetic field.34 SPR bands were centered at 440 nm. The bands were broad and the intensity increased with time, indicating an increase in production of nanoparticles with temperature. At 70 °C (Fig. S3†), the plasmon intensity of the reaction at 65 minutes was near that at 90 minutes. Similarly, at 100 °C (Fig. 2B), the SPR at 45 minutes was near that at 55 minutes. This signifies the completion of the reaction. It is possible to determine the efficiency as a function of time using normalized UV-vis spectra. Fig. 2C shows the time-dependent conversion of Ag+ to AgNPs at 100 °C. After 35 minutes, the reaction levelled off at 89% efficiency.
3.4 Morphological investigation
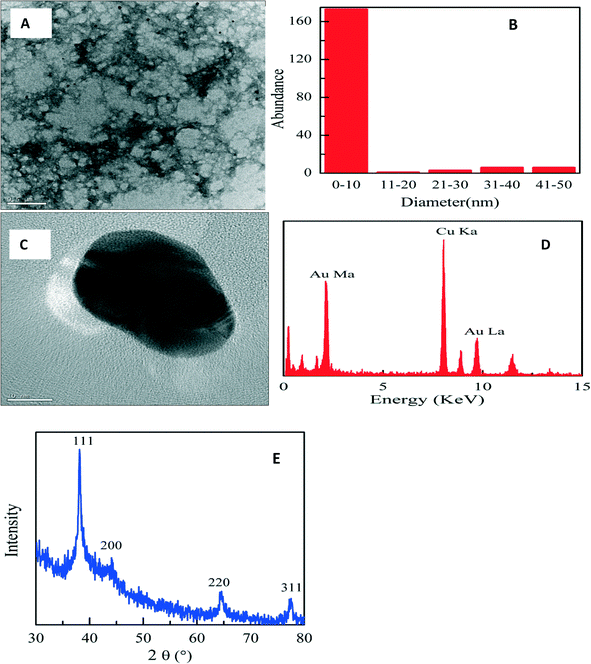 | ||
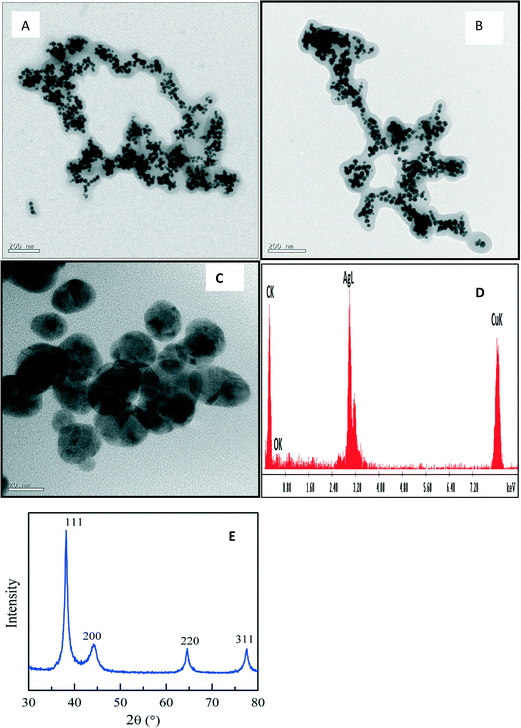
| Fig. 3 TEM micrograph (A), particle size histogram (B), high-resolution TEM micrograph (C), EDX spectrum (D), and XRD patterns (E) of PAA-stabilized AuNP. | ||
Fig. 3E shows the XRD studies of the PAA-stabilized nanoparticles which displayed four diffraction peaks at 38.12°, 43.07°, 64.47°, and 77.22° that can be indexed to the (111), (200), (220), and (311) planes of fcc gold crystallites, respectively. The size of the crystallites along the [111] direction was estimated to be 9.7 ± 0.5 nm based on the calculation using the Scherrer equation. The XRD data are in good agreement with the TEM result in Fig. 3A.
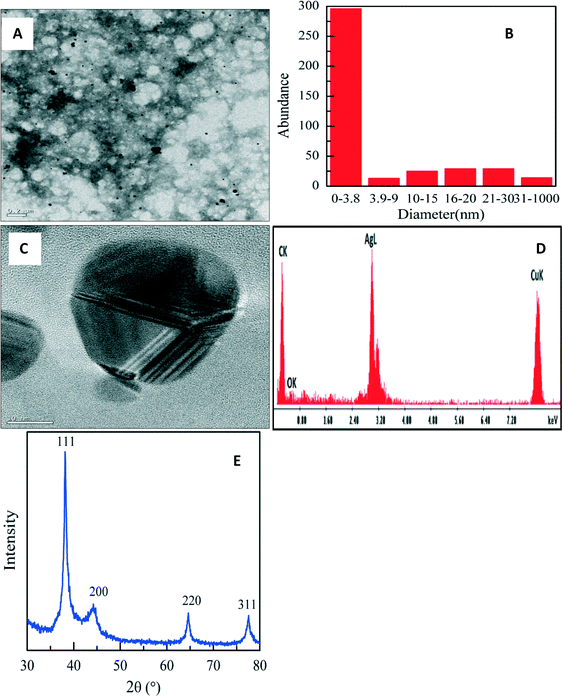 | ||
| Fig. 4 TEM micrograph (A), size distribution histogram (B), high resolution TEM micrograph (C), EDS spectrum (D) and XRD pattern (E) of PAA-stabilized AgNPs at room temperature. | ||
At 70 °C there was no significant change in the size distribution (Fig. S4†) as AgNPs of 4 nm size were formed. Particles were also highly crystalline as seen in the XRD, having FCC patterns with diffraction peaks (Fig. S4) that were indexed to (111), (200), (220) and (311).
At 100 °C, PAA–AgNP composites were formed with dispersed AgNPs trapped within the polymer matrix as shown in Fig. 5A and B. A high-resolution TEM micrograph (Fig. 5C) revealed highly crystalline overlapping particles with an fcc pattern as confirmed by the XRD pattern (Fig. 5E).
3.5 Catalytic activity of PAA-stabilized AuNPs
As stated earlier, an effective way to prevent aggregation is to utilize appropriate stabilizers. However, it has been shown that the presence of a densely packed capping layer on the metal nanoparticle surface may compromise the catalytic activity since surface active sites become inaccessible.8,10,17,35 Therefore, it was necessary to confirm whether the AuNPs stabilized with PAA retained their catalytic activity. The reduction of 4-nitrophenol into 4-aminophenol in the presence of excessive NaBH4 was chosen as a model to test the catalytic activity of the PAA-stabilized AuNPs. This reaction has been used extensively as a benchmark to quantify the catalytic activity of various metallic nanoparticles since the reaction dynamics can be easily monitored using UV-vis absorption spectroscopy.17,36 Upon the addition of excessive NaBH4 into a 4-nitrophenol solution, the color of the solution changed quickly from light yellow to green yellow.Fig. 6a depicts the UV-vis absorption spectra of 4-nitrophenol solution in the presence of PAA-stabilized AuNPs collected at different reaction intervals. At the beginning of the reaction, the solution exhibited a major absorption peak at 400 nm and another band at 300 nm. This is due to the formation of 4-nitrophenolate anions under basic conditions. The intensity of the peak at 400 nm decreased quickly with time, and at the same time the intensity of the peak at 300 nm increased. The rate of reduction is monitored by the disappearance of this peak. The UV-vis spectra show an isosbestic point (around 320 nm), suggesting that the catalytic reduction of 4-nitrophenol gives 4-aminophenol only, without by-product. The reaction appeared to complete within 500 s. The accompanying color change is shown in Fig. S5.† The absorption peak at 300 nm is characteristic of 4-aminophenol.36,37 The graph clearly indicates the effective reduction of 4-nitrophenol into 4-aminophenol in the presence of PAA-synthesized AuNPs.
Since this reaction was conducted under a large excess of NaBH4, the reaction rate was nearly independent of the NaBH4 concentration and consequently the kinetics can be modeled by a quasi-first order process with respect to the concentration of 4-nitrophenol. Therefore the rate constant for the reduction process was determined by measuring the change in absorbance at 400 nm as a function of time. Fig. 6C depicts the plot of ln(At/A0) as a function of reaction time (t), where At and A0 are absorption intensities at time t and 0, respectively. The kinetic rate constant (k) of 5.2 × 10−3 s−1 was derived from the linear regression of the experimental data.
An additional control experiment was set up by adding only NaBH4 to 4-nitrophenol but without PAA-stabilized gold nanoparticles. The UV-vis absorption profiles remained unchanged for up to 60 minutes, indicating that no reduction of 4-nitrophenol into 4-aminophenol occurred in the absence of PAA-stabilized gold nanoparticles (Fig. 6B).
3.6 Catalytic activity of PAA-synthesized AgNPs
The reduction of methylene blue (MB) has been used extensively as a benchmark to quantify the catalytic activity of various metallic nanoparticles since the reaction dynamics can be easily monitored using UV-vis absorption.38,39 The addition of PAA-synthesized AgNPs and excess of 32 mM NaBH4 into MB solution at room temperature produced a color change from blue to colorless. MB exhibits a peak maxima at 658 nm, which corresponds to n–π* transition, and a shoulder at 614 nm.40,41 In the presence of NaBH4, the reaction barely takes place as shown by the constant intensity of the absorption band at 664 nm (Fig. S7A†). However in the presence of AgNPs, MB undergoes a faster reduction to form leucomethylene blue (Fig. S6 and S7B†), and the methylene blue absorption peak at 664 nm decreases rapidly within 3 minutes. This confirms the catalytic activity of the PAA-synthesized AgNPs. The first-order kinetics modeling reveals a rate constant of 1.09 × 10−2 s−1.3.7 Antimicrobial activity and cytotoxicity of PAA-stabilized silver nanoparticles
As detailed in Fig. 7, AgNPs showed antibacterial activity on Escherichia coli DH5 Alpha (Gram negative) and Listeria monocytogenes strains F2365 and HCC7 (Gram positive) as well as on E. coli 25922 and S. epidermidis (results are shown in the ESI,† Fig. S9), Aeromonas hydrophila (Gram negative) and Pseudomonas aeruginosa (Gram negative) (results are shown in the ESI,† Fig. S11). Unlike the response of E. coli 25922 (Gram negative) and S. epidermidis (Gram positive) to silver nanoparticles, Escherichia coli DH5 Alpha and Listeria monocytogenes (strains F2365 and HCC7) showed different responses to AgNP toxicity. The treatment decreased the E. coli DH5 colony count by 75% in comparison to the control, while the treatment of Listeria monocytogenes (F2365 and HCC7) colonies with AgNPs decreased their sizes by over 35%.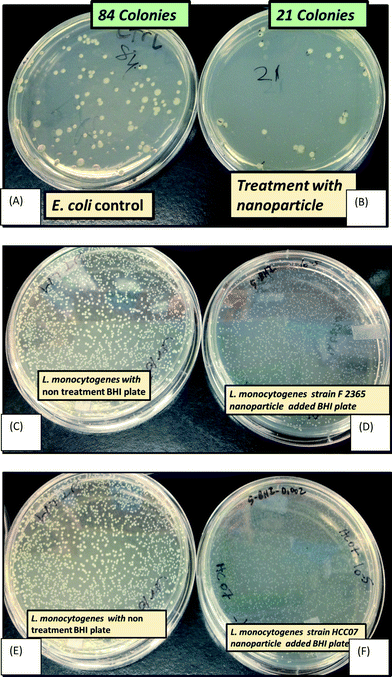 | ||
| Fig. 7 Growth of E. coli upon treatment with 10 μg mL−1 silver nanoparticle agar (B), and control non-treated LB plate (A). Antibacterial activity recorded for L. monocytogenes strain F2365 upon treatment with 10 μg mL−1 nanoparticle agar: (D) and (C) control LB plate; and L. monocytogenes strain HCC07 (F) and control non-treated plate (E). For more details on this setup, refer to S11, ESI. † | ||
However, in all cases AgNPs exhibited antibacterial activity against these Gram (+) and Gram (−) bacteria. The difference of how AgNPs showed their anti-bacterial activities can be explained in three ways: (i) type of bacteria, (ii) medium differences and/or (iii) age and concentration of microbial inoculums. However, it should be mentioned that the age of the silver nanoparticles also made a difference in addition to these three reasons. One week old silver nanoparticles were tested for E. coli 25922 and S. epidermidis, and it was observed that the antibacterial activity decreased by about 10% within 3 months (data not shown); the results presented here are from 3 month old nanoparticles. Different concentrations of silver nanoparticles did not show dramatic changes because autoclaving AgNPs caused agglomeration, which was not seen before autoclaving. The AgNPs did not show even distribution after 1 μg mL−1 concentration, which is shown in the ESI† (Fig. S12).
In summary, the results clearly showed that the synthesized silver nanoparticles exhibit strong antibacterial activity through either decreasing the colony number or reducing the size of formed colonies for bacteria [detailed in Fig. S9†] and slight inhibitory effects on fungi (Fig. S8†).
All cytotoxicity results are displayed in the ESI† (Fig. S10). Fig. S10A and B† depict a change in growth pattern of Caco-2 cells in response to AgNP treatment while IEC-6 cells showed a 5% decrease in confluence. Fig. S10E† shows a fluorescence-based viability study: AgNP concentration above 5 μg mL−1 resulted in over 25% decrease in viability. This shows that the AgNPs exhibited mild cytotoxicity against non-cancerous IEC-6 cells.
4. Conclusion
Herein we have reported a one-step method for the synthesis of well-dispersed AuNPs and AgNPs using PAA as both the reductant and the stabilizer. The as-prepared nanoparticles were stable for over 3 months at room temperature. The nanoparticles formed had a narrow size distribution (~10 nm for AuNPs and ~5 nm for AgNPs). Further temperature dependence of the formation of AgNPs revealed that PAA-AgNP nanocomposites can be synthesized at 100 °C without altering the fcc crystal pattern. The utilization of a biodegradable polymer eliminates the use of toxic volatile organics during the synthetic process. We have also shown that the as-prepared PAA-stabilized AuNPs and AgNPs retained their catalytic activity towards 4-nitrophenol and methylene blue with a rate constant of 5.2 × 10−3 s−1 and 1.09 × 10−2 s−1, respectively. Finally, this work has also demonstrated that the AgNPs exhibited robust antimicrobial activity against Escherichia coli, Staphylococcus epidermidis, Aeromonas hydrophila, Pseudomonas aeruginosa, and Listeria monocytogenes and modest cytotoxicity against non-cancerous immortalized IEC-6 and cancerous Caco-2 cell lines.Acknowledgements
The authors acknowledge the National Science Foundation CBET 1230189 for funding. Idris Yazgan and Ali Akgul also acknowledge the support of a Turkish Government fellowship. The Regional NMR facility (600 MHz instrument) at SUNY-Binghamton is supported by the NSF (CHE-0922815).References
- S. Andreescu, J. Njagi, C. Ispas and M. T. Ravalli, J. Environ. Monit., 2009, 11, 27–40 RSC.
- I. Pastoriza-Santos and L. M. Liz-Marzán, Nano Lett., 2002, 2, 903–905 CrossRef CAS.
- T. K. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648–8649 CrossRef CAS.
- R. A. Sperling, P. R. Gil, F. Zhang, M. Zanella and W. J. Parak, Chem. Soc. Rev., 2008, 37, 1896–1908 RSC.
- S. Guo and E. Wang, Anal. Chim. Acta, 2007, 598, 181–192 CrossRef CAS.
- D. Pissuwan, T. Niidome and M. B. Cortie, J. Controlled Release, 2011, 149, 65–71 CrossRef CAS.
- L. Dykman and N. Khlebtsov, Chem. Soc. Rev., 2012, 41, 2256–2282 RSC.
- R. M. Crooks, M. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181–190 CrossRef CAS.
- R. Su, R. Tiruvalam, Q. He, N. Dimitratos, L. Kesavan, C. Hammond, J. A. Lopez-Sanchez, R. Bechstein, C. J. Kiely and G. J. Hutchings, ACS Nano, 2012, 6, 6284–6292 CrossRef CAS.
- M. M. Nigra, J.-M. Ha and A. Katz, Catal. Sci. Technol., 2013, 3, 2976–2983 CAS.
- S. M. Marinakos, D. A. Shultz and D. L. Feldheim, Adv. Mater., 1999, 11, 34–37 CrossRef CAS.
- D. W. Hatchett, M. Josowicz, J. Janata and D. R. Baer, Chem. Mater., 1999, 11, 2989–2994 CrossRef CAS.
- K. Mallick, M. J. Witcomb and M. S. Scurrell, J. Mater. Sci., 2006, 41, 6189–6192 CrossRef CAS.
- H. Gu, S. Tadakamalla, Y. Huang, H. A. Colorado, Z. Luo, N. Haldolaarachchige, D. P. Young, S. Wei and Z. Guo, ACS Appl. Mater. Interfaces, 2012, 4, 5613–5624 CAS.
- X. Feng, H. Huang, Q. Ye, J.-J. Zhu and W. Hou, J. Phys. Chem. C, 2007, 111, 8463–8468 CAS.
- S. Panigrahi, S. Basu, S. Praharaj, S. Pande, S. Jana, A. Pal, S. K. Ghosh and T. Pal, J. Phys. Chem. C, 2007, 111, 4596–4605 CAS.
- X. Liu, L. Li, M. Ye, Y. Xue and S. Chen, Nanoscale, 2014, 6, 5223–5229 RSC.
- N. Du, C. Wong, M. Feurstein, O. A. Sadik, C. Umbach and B. Sammakia, Langmuir, 2010, 26, 14194–14202 CrossRef CAS.
- D. Andreescu, A. K. Wanekaya, O. A. Sadik and J. Wang, Langmuir, 2005, 21, 6891–6899 CrossRef CAS.
- N. M. Noah, M. Omole, S. Stern, S. Zhang, O. A. Sadik, E. H. Hess, J. Martinovic, P. G. Baker and E. I. Iwuoha, Anal. Biochem., 2012, 428, 54–63 CrossRef CAS.
- I. Yazgan, N. Du, R. Congdon, V. Okello and O. A. Sadik, J. Membr. Sci., 2014, 261–271 CrossRef CAS.
- O. Sadik, I. Yazgan and V. Kariuki, Chemical Processes for a Sustainable Future, 2014, p. 259 Search PubMed.
- M. A. Omole, V. A. Okello, V. Lee, L. Zhou, O. A. Sadik, C. Umbach and B. Sammakia, ACS Catal., 2011, 1, 139–146 CrossRef CAS.
- V. A. Okello, N. Du, B. Deng and O. A. Sadik, J. Environ. Monit., 2011, 13, 1236–1245 RSC.
- K. Neoh, K. Tan, P. Goh, S. Huang, E. Kang and K. Tan, Polymer, 1999, 40, 887–893 CrossRef CAS.
- S. Huang, K. Neoh, E. Kang, H. Han and K. Tan, J. Mater. Chem., 1998, 8, 1743–1748 RSC.
- J. Shan and H. Tenhu, Chem. Commun., 2007, 4580–4598 RSC.
- M. El-Rafie, M. El-Naggar, M. Ramadan, M. M. Fouda, S. S. Al-Deyab and A. Hebeish, Carbohydr. Polym., 2011, 86, 630–635 CrossRef CAS.
- S. S. Shankar, A. Rai, A. Ahmad and M. Sastry, J. Colloid Interface Sci., 2004, 275, 496–502 CrossRef CAS.
- S. Manna, S. K. Batabyal and A. K. Nandi, J. Phys. Chem. B, 2006, 110, 12318–12326 CrossRef CAS.
- J. AlanáCreighton, J. Chem. Soc., Faraday Trans., 1991, 87, 3881–3891 RSC.
- A. Henglein and D. Meisel, Langmuir, 1998, 14, 7392–7396 CrossRef CAS.
- R. R. Naik, S. J. Stringer, G. Agarwal, S. E. Jones and M. O. Stone, Nat. Mater., 2002, 1, 169–172 CrossRef CAS.
- P. Mulvaney, Langmuir, 1996, 12, 788–800 CrossRef CAS.
- J. A. Lopez-Sanchez, N. Dimitratos, C. Hammond, G. L. Brett, L. Kesavan, S. White, P. Miedziak, R. Tiruvalam, R. L. Jenkins and A. F. Carley, Nat. Chem., 2011, 3, 551–556 CrossRef CAS.
- X. Zhang and Z. Su, Adv. Mater., 2012, 24, 4574–4577 CrossRef CAS.
- D. M. Dotzauer, J. Dai, L. Sun and M. L. Bruening, Nano Lett., 2006, 6, 2268–2272 CrossRef CAS.
- V. Vidhu and D. Philip, Micron, 2014, 56, 54–62 CrossRef CAS.
- C. Corredor, M. D. Borysiak, J. Wolfer, P. Westerhoff and J. D. Posner, Environ. Sci. Technol., 2015, 49, 3611–3618 CrossRef CAS.
- T. Shahwan, S. Abu Sirriah, M. Nairat, E. Boyacı, A. Eroğlu, T. Scott and K. Hallam, Chem. Eng. J., 2011, 172, 258–266 CrossRef CAS.
- M. A. Rauf, M. A. Meetani, A. Khaleel and A. Ahmed, Chem. Eng. J., 2010, 157, 373–378 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S11 provide additional information on the synthesis, characterization, catalytic and antimicrobial activity, and cytotoxicity of the PAA-synthesized AgNPs and AuNPs. See DOI: 10.1039/c5en00053j |
| This journal is © The Royal Society of Chemistry 2015 |