Themed collection Aromaticity

Aromaticity: a web themed issue
Showcasing a collection of cutting edge contributions by international leaders in the field of aromaticity.

Chem. Commun., 2012,48, 10471-10471
https://doi.org/10.1039/C2CC90288E
Molecular early main group metal hydrides: synthetic challenge, structures and applications
Ligand-stabilized early main group metal hydride clusters.
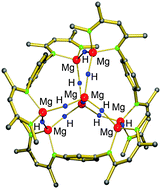
Chem. Commun., 2012,48, 11165-11177
https://doi.org/10.1039/C2CC33478J
Synthesis of π-extended porphyrins via intramolecular oxidative coupling
Fusion of porphyrins with other aromatic units at the meso- and β-positions results in very strong bathochromic shifts of absorption.
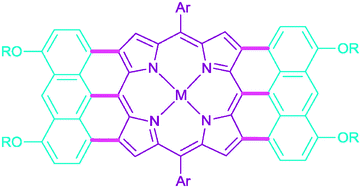
Chem. Commun., 2012,48, 10069-10086
https://doi.org/10.1039/C2CC31279D
Coarctate cyclization reactions: a primer
Coarctate reactivity, typical of molecules in the conjugated ‘ene–ene–yne’ manifold, can be harnessed to generate unique classes of heterocyclic compounds.

Chem. Commun., 2012,48, 9441-9455
https://doi.org/10.1039/C2CC34026G
The chemistry of four-membered aromatics
This feature article overviews and summarizes aromaticity of the four-membered ring systems to discuss expanding chemistry of the aromaticity concept.
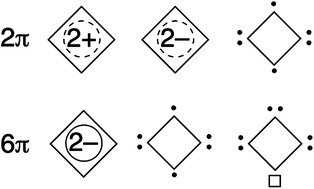
Chem. Commun., 2012,48, 9334-9342
https://doi.org/10.1039/C2CC34244H
Oxidative transformation of aryls using molybdenum pentachloride
Aryl–aryl bonds can effectively be formed using molybdenum pentachloride to obtain uncommon geometries tolerating a broad scope of functional groups.
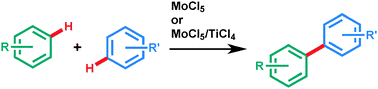
Chem. Commun., 2012,48, 9109-9119
https://doi.org/10.1039/C2CC33925K
Tunable star-shaped triphenylamine fluorophores for fluorescence quenching detection and identification of nitro-aromatic explosives
Triphenylamine-based fluorophores containing pyrene or corannulene show variable fluorescence quenching sensitivity toward nitro explosives.
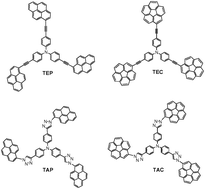
Chem. Commun., 2013,49, 780-782
https://doi.org/10.1039/C2CC34008A
The 4,5,6-triphospha[3]radialene dianion: a phosphorus analogue of the deltate dianion. A NICS(0)πzz examination of their aromaticity
Reduction of a 4,5,6-triphospha[3]radialene gave the corresponding isolable dianionic species.
![Graphical abstract: The 4,5,6-triphospha[3]radialene dianion: a phosphorus analogue of the deltate dianion. A NICS(0)πzz examination of their aromaticity](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CC35978B&imageInfo.ImageIdentifier.Year=2012)
Chem. Commun., 2012,48, 11440-11442
https://doi.org/10.1039/C2CC35978B
Unusual para-substituent effects on the intramolecular hydrogen-bond in hydrazone -based switches
A “V”-shaped Hammett plot shows that resonance-assisted hydrogen bonding does not dictate the strength of the intramolecular hydrogen bond in the E isomers of hydrazone-based switches.
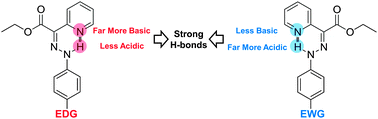
Chem. Commun., 2012,48, 10490-10492
https://doi.org/10.1039/C2CC35860C
On the aromatic stabilization of benzenoid hydrocarbons
We present a general scheme for estimation of aromatic stabilization energies of benzenoid hydrocarbons based on selected topological features.
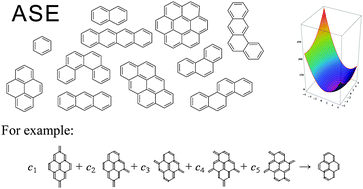
Chem. Commun., 2012,48, 10129-10131
https://doi.org/10.1039/C2CC33974A
Molecular nesting in co-crystals of tetrabenzoquadrannulene and C60: application of the sphere in a cone model
The interaction between C60 and tetrabenzoquadrannulene, observed by X-ray diffraction analysis, is understood using DFT-D calculations and a sphere-in-cone model.
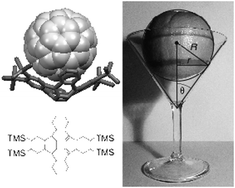
Chem. Commun., 2012,48, 9882-9884
https://doi.org/10.1039/C2CC34472F
Highly fluorescent benzofuran derivatives of the GFP chromophore
Intramolecular cyclization of alkynyl-substituted derivatives of the Green Fluorescent Protein chromophore into benzofuran derivatives presents a simple way of enhancing its fluorescence.
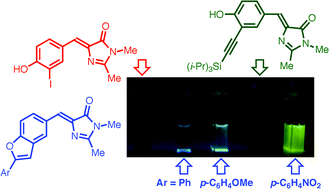
RSC Adv., 2012,2, 8243-8249
https://doi.org/10.1039/C2RA21380J
π-Depletion as a criterion to predict π-stacking ability
The LOLIPOP criterion identifies π-depleted polyaromatic molecules, which exhibit enhanced π-stacking ability.
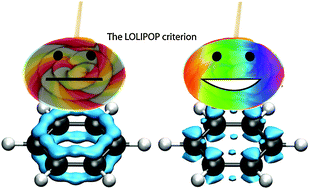
Chem. Commun., 2012,48, 9239-9241
https://doi.org/10.1039/C2CC33886F
Synthesis of 3,7-diiodo-2,6-di(thiophen-2-yl)benzo[1,2-b:4,5-b′]difurans: functional building blocks for the design of new conjugated polymers
Iodine-catalyzed cyclization is used to prepare benzodifurans that can be further functionalized to alter their properties.
![Graphical abstract: Synthesis of 3,7-diiodo-2,6-di(thiophen-2-yl)benzo[1,2-b:4,5-b′]difurans: functional building blocks for the design of new conjugated polymers](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CC34070D&imageInfo.ImageIdentifier.Year=2012)
Chem. Commun., 2012,48, 8919-8921
https://doi.org/10.1039/C2CC34070D
Merging tribenzotriquinacene with hexa-peri-hexabenzocoronene : a cycloheptatriene unit generated by Scholl reaction
A bay-bridging Scholl reaction converts the pentaphenylphenyl-substituted tribenzotriquinacene 1 into the curved hexa-peri-hexabenzocoronene-TBTQ derivative 2 bearing a newly formed cycloheptatriene ring.

Chem. Commun., 2012,48, 8880-8882
https://doi.org/10.1039/C2CC34245F
Highly π electron-rich macro-aromatics: bis(p-aminophenyl)-carbo-benzenes and their DBA acyclic references
A series of quadrupolar bis(p-aminophenyl)-carbo-benzenes (bottom) is compared to the acyclic carbo-butadiene (top) reference series.
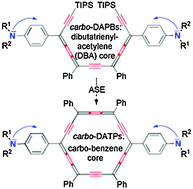
Chem. Commun., 2012,48, 8763-8765
https://doi.org/10.1039/C2CC34176J
A small molecule two-photon probe for hydrogen sulfide in live tissues
We report a two-photon probe (FS1) which shows a 21-fold two-photon excited fluorescence enhancement in response to H2S and can selectively detect H2S in a rat hippocampal slice at a depth of 90–190 μm by using two-photon microscopy.
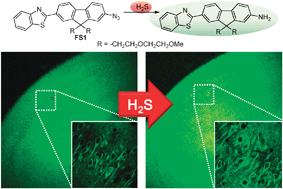
Chem. Commun., 2012,48, 8395-8397
https://doi.org/10.1039/C2CC33909A
Is cyclobutadiene really highly destabilized by antiaromaticity?
The high energy of cyclobutadiene (CBD) is not due primarily to “anti-aromaticity,” but rather to angle strain and Pauli repulsion between close-by parallel CC σ and π bonds.
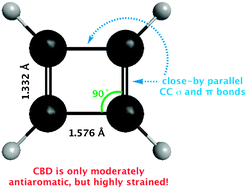
Chem. Commun., 2012,48, 8437-8439
https://doi.org/10.1039/C2CC33521B
Dibenz[a,c]anthracene derivatives exhibiting columnar mesophases over broad temperature ranges
The mesomorphic properties of a series of novel dibenz[a,c]anthracenes display remarkable sensitivity to substituents and in some cases these compounds exhibit mesophases over broad temperature ranges.
![Graphical abstract: Dibenz[a,c]anthracene derivatives exhibiting columnar mesophases over broad temperature ranges](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CC33407K&imageInfo.ImageIdentifier.Year=2012)
Chem. Commun., 2012,48, 8210-8212
https://doi.org/10.1039/C2CC33407K
Core-extended rylene dyes via thiophene annulation
Annulation of thiophenes directly into the bay regions of rylene dyes leads to core-extended rylene tetracarboxylic diimides with interesting electro-optical properties.
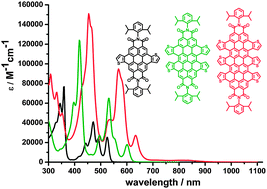
Chem. Commun., 2012,48, 8204-8206
https://doi.org/10.1039/C2CC33529H
Synthesis and charge transport studies of stable, soluble hexacenes
We report the synthesis and charge transport characterization of soluble hexacenes, including ambipolar behavior from an octafluoro hexacene.
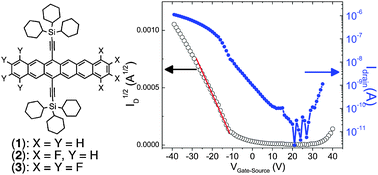
Chem. Commun., 2012,48, 8261-8263
https://doi.org/10.1039/C2CC33919F
An experimental estimate of the relative aromaticity of the cyclooctatetraene dianion by fusion to dimethyldihydropyrene
The relative aromaticity of COT2− is experimentally as large as benzene, and larger than that of the cyclopentadienide anion.

Chem. Commun., 2012,48, 8144-8146
https://doi.org/10.1039/C2CC33518B
Palladium -catalysed heteroannulation of [60]fullerene with N-benzyl sulfonamides and subsequent functionalisation
The palladium-catalysed heteroannulation of [60]fullerene with N-benzyl sulfonamides via C–H activation affords [60]fullerene-fused tetrahydroisoquinolines, which can be further functionalised.
![Graphical abstract: Palladium-catalysed heteroannulation of [60]fullerene with N-benzyl sulfonamides and subsequent functionalisation](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CC33923D&imageInfo.ImageIdentifier.Year=2012)
Chem. Commun., 2012,48, 8132-8134
https://doi.org/10.1039/C2CC33923D
Synthesis of electron-poor hexa-peri-hexabenzocoronenes
A series of hexa-peri-hexabenzocoronenes with electron withdrawing groups were obtained via oxidative cyclodehydrogenation with an oxidant and a strong acid.
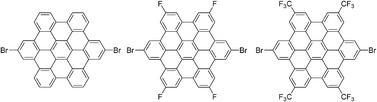
Chem. Commun., 2012,48, 8066-8068
https://doi.org/10.1039/C2CC33892K
Diels–Alder cycloaddition of acetylene gas to a polycyclic aromatic hydrocarbon bay region
Acetylene might suffice as a dienophile for growing uniform carbon nanotubes from small hydrocarbon templates by the Diels–Alder cycloaddition/rearomatization strategy.
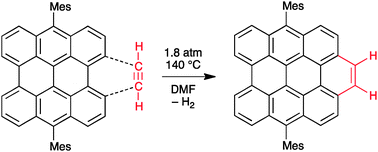
Chem. Commun., 2012,48, 8102-8104
https://doi.org/10.1039/C2CC33885H
Chemically modified graphene oxides as a hole transport layer in organic solar cells
O2 plasma treated GO with a work function of 5.2 eV as a high performance hole transport layer in organic solar cells.
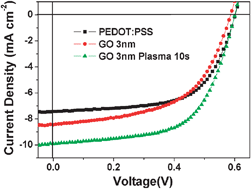
Chem. Commun., 2012,48, 8078-8080
https://doi.org/10.1039/C2CC33829G
Relationship of charge to antiaromaticity in bis-fluorenyl dications and fluorenyl monocations: experimental support for theoretical calculations
Antiaromaticity of fluorenyl cations can be moderated through the use of substituents to change the amount of charge density.
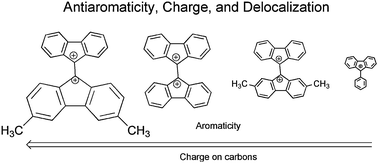
Chem. Commun., 2012,48, 8099-8101
https://doi.org/10.1039/C2CC33799A
Synthesis and structure of 1,4,5,8-tetraethynylnaphthalene derivatives
1,4,5,8-Tetraethynylnaphthalene derivatives were synthesized for the first time. X-ray crystallographic structure analysis of the tetrakis(phenylethynyl) substituted derivative revealed three different modes of distortion to reduce steric repulsion.
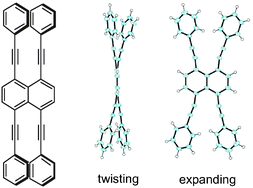
Chem. Commun., 2012,48, 7841-7843
https://doi.org/10.1039/C2CC33740A
Tetrakis(4-tert-butylphenyl) substituted and fused quinoidal porphyrins
Tetrakis(4-tert-butylphenyl) substituted and fused quinoidal porphyrins are prepared for the first time, which show unique geometry, electronic structure and optical properties.
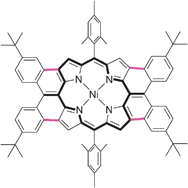
Chem. Commun., 2012,48, 7684-7686
https://doi.org/10.1039/C2CC33728B
Concise, aromatization-based approach to an elaborate C2-symmetric pyrenophane
A multicomponent reaction and a double McMurry/valence isomerization/dehydrogenation reaction conspire to create the entire pyrene system in a highly decorated [12](1,6)pyrenophane.
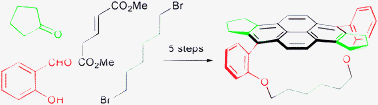
Chem. Commun., 2012,48, 7747-7749
https://doi.org/10.1039/C2CC33611A
Straightforward synthesis of phenanthrenes from styrenes and arenes
A semi-one-pot synthesis of phenanthrenes from styrenes and arenes has been developed which provides an effective and practical protocol.
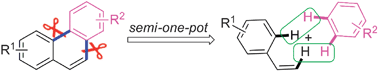
Chem. Commun., 2012,48, 7028-7030
https://doi.org/10.1039/C2CC33100D
A rigid donor–acceptor daisy chain dimer
A functionalised cyclobis(paraquat-p-phenylene) attached by a rigid linker to a tetrathiafulvalene unit, which is incapable of self-complexation, forms preferentially a [c2]daisy chain which undergoes rapid disassociation and reassociation on the 1H NMR time-scale above room temperature.
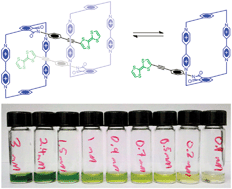
Chem. Commun., 2012,48, 6791-6793
https://doi.org/10.1039/C2CC32499G
α-Oligofurans show a sizeable extent of π-conjugation as probed by Raman spectroscopy
Raman spectroscopic analysis of long α-oligofurans and polyfuran shows that extension of the π-conjugation in polyfuran spreads over 14–15 furan units.
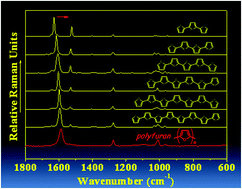
Chem. Commun., 2012,48, 6732-6734
https://doi.org/10.1039/C2CC18144D
1,3,5-Triaryl 2-pyridylidene: base-promoted generation and complexation
We herein report a base-promoted generation and complexation of sterically hindered 1,3,5-triaryl 2-pyridylidene from the corresponding pyridinium salt.
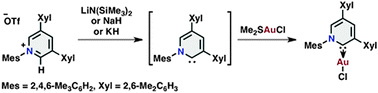
Chem. Commun., 2012,48, 6642-6644
https://doi.org/10.1039/C2CC33184E
Meta-B-entacenes: new polycyclic aromatics incorporating two fused borepin rings
New, curved boron-containing acenes have been synthesized, demonstrating cathodic electrochemistry, strong photoluminescence, and facile cross-coupling to give π-extended scaffolds.
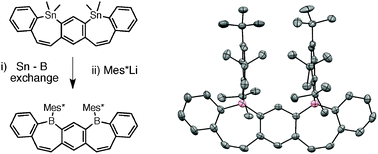
Chem. Commun., 2012,48, 6256-6258
https://doi.org/10.1039/C2CC32500D
A C84 selective porphyrin macrocycle with an adaptable cavity constructed through alkyne metathesis
A bisporphyrin macrocycle with an adaptable cavity showing a high binding selectivity for C84 over lower fullerenes (C60 and C70).
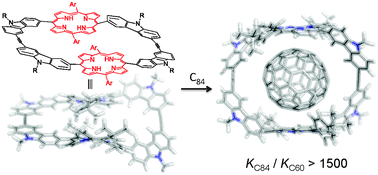
Chem. Commun., 2012,48, 6172-6174
https://doi.org/10.1039/C2CC32571C
Angular-shaped naphthodifurans, naphtho[1,2-b;5,6-b′]- and naphtho[2,1-b;6,5-b′]-difuran: are they isoelectronic with chrysene?
The chalcogen atoms make a marked difference in the ground state electronic structure.
![Graphical abstract: Angular-shaped naphthodifurans, naphtho[1,2-b;5,6-b′]- and naphtho[2,1-b;6,5-b′]-difuran: are they isoelectronic with chrysene?](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CC31546G&imageInfo.ImageIdentifier.Year=2012)
Chem. Commun., 2012,48, 5671-5673
https://doi.org/10.1039/C2CC31546G
Aromaticity and π-bond covalency: prominent intermolecular covalent bonding interaction of a Kekulé hydrocarbon with very significant singlet biradical character
A prominent covalent bonding interaction between molecules is experimentally elucidated in a one-dimensional stack of an anthracene-linked bisphenalenyl Kekulé molecule by using polarized reflection spectroscopy.
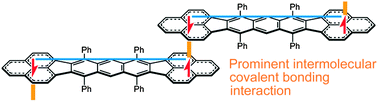
Chem. Commun., 2012,48, 5629-5631
https://doi.org/10.1039/C2CC31955A
Diazaheterocycle analogues of tetracene : synthesis and properties of a naphtho-fused cinnoline and a naphtho-fused isoindazole
Two fluorescent, diaza-analogues of tetracene can be prepared independently from a common triazene-ene-yne precursor via either a pericyclic or coarctate ring-forming reaction.
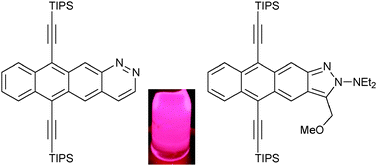
Chem. Commun., 2012,48, 5166-5168
https://doi.org/10.1039/C2CC31613G
Rearrangement from the heteroantiaromatic borole to the heteroaromatic azaborine motif
Treatment of 9-chloro-9-borafluorene with N,O-(bistrimethylsilyl)hydroxylamine produces an azaborine derivative.

Chem. Commun., 2012,48, 4564-4566
https://doi.org/10.1039/C2CC30914A
Deterioration of bulk heterojunction organic photovoltaic devices by a minute amount of oxidized fullerene
Addition of SIMEF-O2 to the active layer of organic photovoltaic devices decreased their performance.
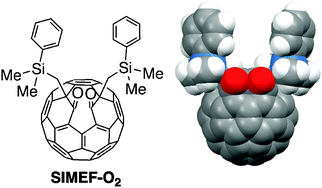
Chem. Commun., 2012,48, 3878-3880
https://doi.org/10.1039/C2CC30262D
Reversible dispersion and releasing of single-walled carbon nanotubes by a stimuli-responsive TTFV-phenylacetylene polymer
A TTFV-phenylacetylene folding polymer exhibits the property of reversibly dispersing and releasing single-walled carbon nanotubes in organic solvents under the control of redox or pH stimuli.
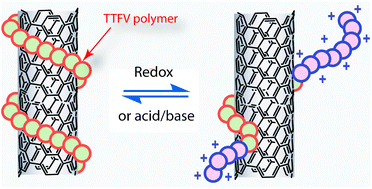
Chem. Commun., 2012,48, 3100-3102
https://doi.org/10.1039/C2CC17935K
Triply ferrocene-bridged boroxine cyclophane
Solvothermal dehydration of 1,1′-ferrocenediboronic acid produces single crystals of a triply ferrocene-bridged boroxine cyclophane.
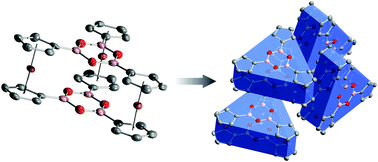
Chem. Commun., 2012,48, 2855-2857
https://doi.org/10.1039/C2CC17744G
The high yielding synthesis of pillar[5]arenes under Friedel–Crafts conditions explained by dynamic covalent bond formation
Systematic investigations on Friedel–Crafts reactions from building blocks A, B and C have been carried out to demonstrate that the formation of pillar[5]arenes occurs under thermodynamic control.
![Graphical abstract: The high yielding synthesis of pillar[5]arenes under Friedel–Crafts conditions explained by dynamic covalent bond formation](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CC16795F&imageInfo.ImageIdentifier.Year=2012)
Chem. Commun., 2012,48, 2576-2578
https://doi.org/10.1039/C2CC16795F
Synthesis of a green [60]fullerene derivative through cage-opening reactions
Open-cage derivative C60(O)4(OH)2(NC6H4tBu)2 reacts with ICl to form a Baeyer–Villiger type product, which yields an intense green product upon treatment with HI.
![Graphical abstract: Synthesis of a green [60]fullerene derivative through cage-opening reactions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CC17164C&imageInfo.ImageIdentifier.Year=2012)
Chem. Commun., 2012,48, 2531-2533
https://doi.org/10.1039/C2CC17164C
About this collection
This web-based themed issue highlights recent achievements in chemistry related to aromaticity. You will find research ranging from new fundamental knowledge about aromaticity and theoretically interesting new arene structures to novel applications of aromatics and heteroaromatics which take advantage of their unique optical and electronic attributes and, in general, on those processes in which the gain or loss of aromaticity play an important role.
The guest editors for this issue are Nazario Martín (University Complutense of Madrid), Michael Haley (University of Oregon) and Rik Tykwinski (University of Erlangen-Nuremberg).
Articles in this web themed issue will be added below as soon as possible after they are published. Please return to this page frequently to see the collection grow.