Abstract
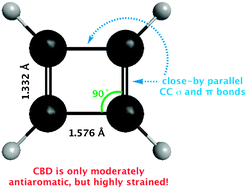
The high energy of cyclobutadiene (CBD) is not due primarily to “anti-aromaticity,” but rather to angle strain, torsional strain, and Pauli repulsion between the parallel CC bonds. Estimations including block-localized wavefunction (BLW) computations conclude that the enormous ring strain (ca. 60 kcal mol−1) far exceeds its antiaromatic destabilization (only 16.5 kcal mol−1).
- This article is part of the themed collection: Aromaticity

 Please wait while we load your content...
Please wait while we load your content...