Themed collection 16th International Conference on Retinal Proteins

The role of the non-covalent β-ionone-ring binding site in rhodopsin: historical and physiological perspective
Rhodopsin regenerates by way of a non-covalent complex formation between the 11-cis retinal and opsin, rendering the β-ionone ring-binding pocket a distinct physiological role.
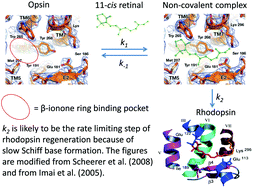
Photochem. Photobiol. Sci., 2015,14, 1932-1940
https://doi.org/10.1039/C5PP00158G
Phospholipid scrambling by rhodopsin
Rhodopsin as phospholipid scramblase – A perspective.
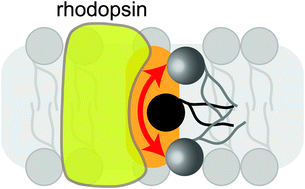
Photochem. Photobiol. Sci., 2015,14, 1922-1931
https://doi.org/10.1039/C5PP00195A
Optimizing optogenetic constructs for control over signaling and cell behaviours
This perspective highlights recent advances in the design of optogenetic tools that provide dynamic subcellular control over signaling and cell behaviour.

Photochem. Photobiol. Sci., 2015,14, 1578-1585
https://doi.org/10.1039/C5PP00171D
Characterizing rhodopsin signaling by EPR spectroscopy: from structure to dynamics
Perspective on how EPR spectroscopy brings insights into rhodopsin and GPCR dynamics.

Photochem. Photobiol. Sci., 2015,14, 1586-1597
https://doi.org/10.1039/C5PP00191A
The regulatory mechanism of ion permeation through a channelrhodopsin derived from Mesostigma viride (MvChR1)
MvChR2 was rather insensitive to Gd3+ because of the absence of negativity at the 116th position, which is glutamate in the case of channelrhodopsin-2 (CrChR2) or the C1C2.
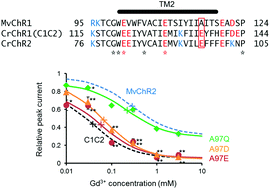
Photochem. Photobiol. Sci., 2016,15, 365-374
https://doi.org/10.1039/C5PP00290G
Rhodopsins carrying modified chromophores – the ‘making of’, structural modelling and their light-induced reactivity
Vitamin-A aldehydes (retinals) with modified alkyl group substituents (9-demethyl-, 9-ethyl-, 9-isopropyl-, 10-methyl, 10-methyl-13-demethyl-, and 13-demethyl retinal) were synthesized to probe the chromophore-proteininteractions in rhodopsin.
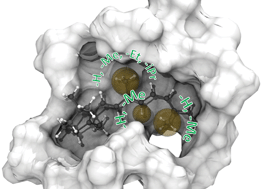
Photochem. Photobiol. Sci., 2016,15, 297-308
https://doi.org/10.1039/C5PP00322A
A light-regulated bZIP module, photozipper, induces the binding of fused proteins to the target DNA sequence in a blue light-dependent manner
Yellow fluorescent protein or mCherry protein fused with the Photozipper underwent blue light-induced dimerization, which enhanced their affinities for the target DNA.
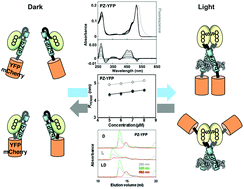
Photochem. Photobiol. Sci., 2015,14, 1998-2006
https://doi.org/10.1039/C5PP00178A
Light-dependent activation of G proteins by two isoforms of chicken melanopsins
A comparative study of non-visual opsins expressed in the chicken pineal gland showed that melanopsins and pinopsins photoactivate mutually different G protein pathways, which regulate melatonin production.

Photochem. Photobiol. Sci., 2015,14, 1991-1997
https://doi.org/10.1039/C5PP00153F
Determination of N-retinylidene-N-retinylethanolamine (A2E) levels in central and peripheral areas of human retinal pigment epithelium
The levels of the bis-retinoid A2E cannot account for the high levels of lipofuscin fluorescence found in the central area of the human retinal pigment epithelium.

Photochem. Photobiol. Sci., 2015,14, 1983-1990
https://doi.org/10.1039/C5PP00156K
Microbial rhodopsins of Halorubrum species isolated from Ejinoor salt lake in Inner Mongolia of China
A halophilic archaea that has three microbial rhodopsin-type genes in its genome was isolated from Ejinoor salt lake in Inner Mongolia of China.

Photochem. Photobiol. Sci., 2015,14, 1974-1982
https://doi.org/10.1039/C5PP00161G
Helical rearrangement of photoactivated rhodopsin in monomeric and dimeric forms probed by high-angle X-ray scattering
The light-induced conformational change of monomeric and dimeric rhodopsin in the nanodisc membrane was directly monitored by high-angle solution X-ray scattering.
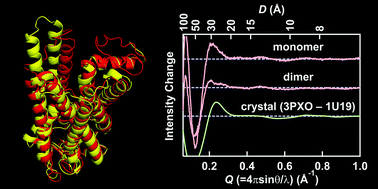
Photochem. Photobiol. Sci., 2015,14, 1965-1973
https://doi.org/10.1039/C5PP00175G
Explaining the mobility of retinal in activated rhodopsin and opsin
Computational studies reveal flexibility of the rhodopsin cofactor, retinal, within the protein binding pocket that play a key role in the activated state and regeneration of rhodopsin.
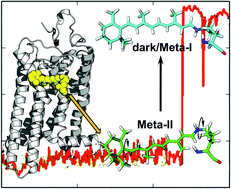
Photochem. Photobiol. Sci., 2015,14, 1952-1964
https://doi.org/10.1039/C5PP00173K
Photo-control of DNA binding by an engrailed homeodomain–photoactive yellow protein hybrid
A photo-controlled version of the engrailed homeodomain (zENG) was created by inserting the homeodomain into a surface loop of a circularly permuted version of the photoactive yellow protein (cPYP).
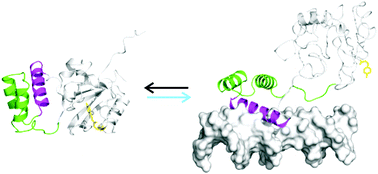
Photochem. Photobiol. Sci., 2015,14, 1729-1736
https://doi.org/10.1039/C5PP00160A
Influence of a chromophore analogue in the protein cage of a photoactive yellow protein
To understand the mechanism of photo-isomerization, photoactive yellow protein (PYP) containing cyano-p-coumaric acid (CHCA) were investigated.
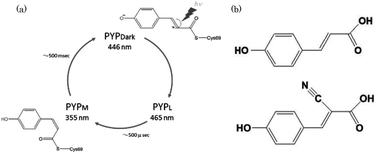
Photochem. Photobiol. Sci., 2015,14, 1722-1728
https://doi.org/10.1039/C5PP00176E
Characterization of photo-intermediates in the photo-reaction pathways of a bacteriorhodopsin Y185F mutant using in situ photo-irradiation solid-state NMR spectroscopy
Photo-reaction pathways of a Y185F-bR mutant and, CS*- and O-intermediates were examined using in situ photo-irradiation solid-state 13C NMR spectroscopy.
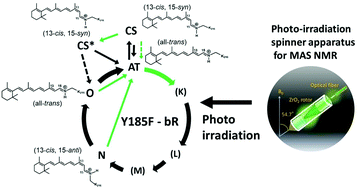
Photochem. Photobiol. Sci., 2015,14, 1694-1702
https://doi.org/10.1039/C5PP00154D
Structural and functional roles of the N- and C-terminal extended modules in channelrhodopsin-1
The extended modules of channelrhodopsin-1 (ChR-1) are involved both in the maintenance of suitable photochemical properties and their functions.
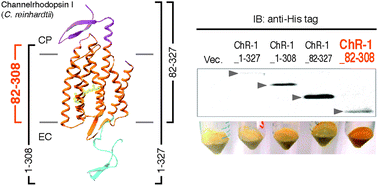
Photochem. Photobiol. Sci., 2015,14, 1628-1636
https://doi.org/10.1039/C5PP00213C
About this collection
The 16th International Conference on Retinal Proteins was held at the Nagahama Royal Hotel in Shiga Japan October 5-10, 2014. The local organizing committee, chaired by Yoshinori Shichida, arranged a very special meeting in a lovely venue. The meeting was attended by scientists, including many students, from across the world. The topics ranged from basic theories as to the function of retinal proteins (including microbial rhodopsins, channelrhodopsins, visual and non-visual rhodopsins as well as a PAS/LOV domain proteins) to the use of these proteins in optogenetics. There was also focus on the cutting edge techniques used in studying the structure and function of these proteins. We include here a selection of papers presented at the meeting. The wide range of topics is an indication of the growth of this field.
Next meeting will be organized by Peter Hegemann and Klaus Gerwert and held at Potsdam Germany in 2016. We hope this will be another occasion to meet and have an excellent opportunity for scientific exchange.
This themed collection is Guest Edited by Professor Rosalie Crouch (Medical University of South Carolina) and Professor Yasushi Imamoto (Kyoto University).