Explaining the mobility of retinal in activated rhodopsin and opsin
Abstract
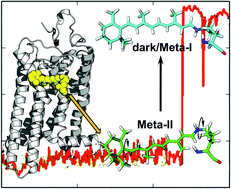
Rhodopsin, the mammalian dim light photoreceptor, is the canonical model for G protein-coupled receptors. Activation of rhodopsin occurs when the covalently bound inverse agonist, retinal, absorbs a photon and undergoes an 11-cis to all-trans isomerization. Two critical components of the visual cycle occur with the (1) hydrolytic release of all-trans retinaldehyde and subsequent (2) uptake of 11-cis retinaldehyde to reform the Schiff base linkage in the apoprotein opsin. Two pores on the surface of opsin are connected via the retinal channel, as discovered upon solution of the X-ray crystal structure (Park et al., Nature, 2008), and could serve as potential entryways for uptake and release. Using molecular dynamics simulations, we examined the behavior of rhodopsin in the Meta-II conformation (active) under Meta-I conditions (inactive), and discovered that the retinal binding pocket is flexible enough to allow a 180° rotation along the long axis of the retinal polyene chain. This result reconciles a discrepancy between the known polyene chain orientation from crystallographic and spectroscopic studies and opens the door for further investigation into the intermolecular interactions between the retinal ligand and the apoprotein opsin. Subsequent docking studies of both isomers of retinal into the opsin channel were then conducted to identify the mechanism for uptake and release. Our results suggest that retinal undergoes unidirectional uptake through Pore A and release through Pore B, and that aromatic sidechain interactions play a key role in stabilizing retinal within the opsin channel. These findings are significant in developing our understanding of the retinoid cycle and how ligand-receptor interactions in rhodopsin relate to G protein-coupled receptor activation.
- This article is part of the themed collection: 16th International Conference on Retinal Proteins

 Please wait while we load your content...
Please wait while we load your content...