Themed collection Biophysical studies on protein misfolding and amyloid diseases

Alzheimer's disease: which type of amyloid-preventing drug agents to employ?
The amyloid hypothesis and toxic ion channel formation in Alzheimer's disease.
Phys. Chem. Chem. Phys., 2013,15, 8868-8877
https://doi.org/10.1039/C3CP00017F
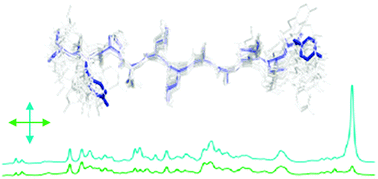
Determination of orientations of aromatic groups in self-assembled peptide fibrils by polarised Raman spectroscopy
In this paper we describe a novel combination of Raman spectroscopy, isotope editing and X-ray scattering as a powerful approach to give detailed structural information on aromatic side chains in peptide fibrils.
Phys. Chem. Chem. Phys., 2013,15, 13940-13950
https://doi.org/10.1039/C3CP52595C
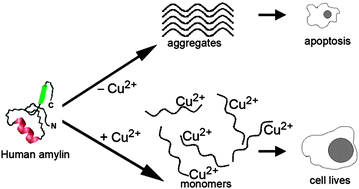
Copper(II)–human amylin complex protects pancreatic cells from amylin toxicity
Cu2+ mitigates aggregation and toxicity of human amylin by stabilizing the peptide in its native, random-coil conformational state.
Phys. Chem. Chem. Phys., 2013,15, 12558-12571
https://doi.org/10.1039/C3CP44542A
Hydrogen bonding involving side chain exchangeable groups stabilizes amyloid quarternary structure
The amyloid β-peptide (Aβ) is the major structural component of amyloid fibrils in the plaques of brains of Alzheimer's disease patients.
Phys. Chem. Chem. Phys., 2013,15, 12551-12557
https://doi.org/10.1039/C3CP44653K
Stereochemical effects on the aggregation and biological properties of the fibril-forming peptide [KIGAKI]3 in membranes
Conformational equilibria of [KIGAKI]3.
![Graphical abstract: Stereochemical effects on the aggregation and biological properties of the fibril-forming peptide [KIGAKI]3 in membranes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3CP50896J&imageInfo.ImageIdentifier.Year=2013)
Phys. Chem. Chem. Phys., 2013,15, 8962-8971
https://doi.org/10.1039/C3CP50896J
Amyloid-β–neuropeptide interactions assessed by ion mobility-mass spectrometry
Small neuropeptides can bind to amyloid-β and modulate their aggregation in vitro.
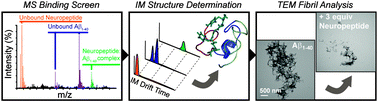
Phys. Chem. Chem. Phys., 2013,15, 8952-8961
https://doi.org/10.1039/C3CP50721A
Analytical model and multiscale simulations of Aβ peptide aggregation in lipid membranes: towards a unifying description of conformational transitions, oligomerization and membrane damage
Twisted arrangements of α-helical Aβ (1–40) peptides are spontaneously assembled in membranes and act as mechanical stressors of the bilayer.
Phys. Chem. Chem. Phys., 2013,15, 8940-8951
https://doi.org/10.1039/C3CP44539A
Initiation of assembly of tau(273-284) and its ΔK280 mutant: an experimental and computational study
The addition of heparin or a point mutation in the segment PHF6* of tau shifts the equilibrium toward more amylogenic conformations.
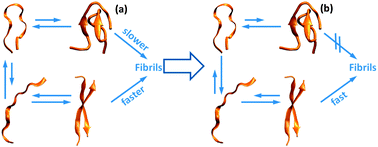
Phys. Chem. Chem. Phys., 2013,15, 8916-8928
https://doi.org/10.1039/C3CP00063J
Role of aromatic residues in amyloid fibril formation of human calcitonin by solid-state 13C NMR and molecular dynamics simulation
Stable fibril structures of hCT in the antiparallel β-sheet were determined by NMR and MD simulation.
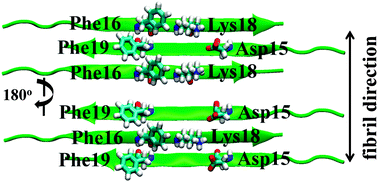
Phys. Chem. Chem. Phys., 2013,15, 8890-8901
https://doi.org/10.1039/C3CP44544E
Cosolvent effects on the fibrillation reaction of human IAPP
Compatible solutes (TMAO) and chaotropic agents (urea) exert different effects on the nucleation and growth process of the fibrils forming islet amyloid polypeptide (IAPP).
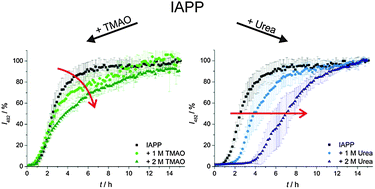
Phys. Chem. Chem. Phys., 2013,15, 8902-8907
https://doi.org/10.1039/C3CP44412K
Membrane disordering is not sufficient for membrane permeabilization by islet amyloid polypeptide : studies of IAPP(20–29) fragments
A key factor in the development of type II diabetes is the loss of insulin-producing beta-cells.
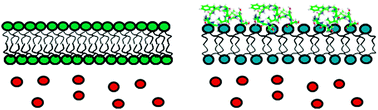
Phys. Chem. Chem. Phys., 2013,15, 8908-8915
https://doi.org/10.1039/C3CP44696D
Effects of membrane interaction and aggregation of amyloid β-peptide on lipid mobility and membrane domain structure
The uptake of GM1 into Aβ aggregates and the attendant membrane damage were microscopically observed.

Phys. Chem. Chem. Phys., 2013,15, 8929-8939
https://doi.org/10.1039/C3CP44517H
Molecular interactions of Alzheimer amyloid-β oligomers with neutral and negatively charged lipid bilayers
Molecular dynamics simulations reveal the differences in orientation, adsorption, surface interaction, and selective ion binding between Aβ–POPC and Aβ–POPC–POPG systems.
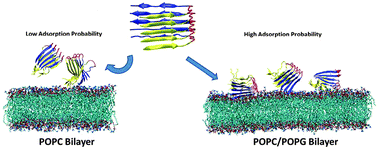
Phys. Chem. Chem. Phys., 2013,15, 8878-8889
https://doi.org/10.1039/C3CP44448A
About this collection
A collection of articles on the theme of protein misfolding and amyloid diseases from a biophysical viewpoint, guest edited by Ayyalusamy Ramamoorthy (University of Michigan).