Direct synthesis of β-acyloxy aldehydes from linear allylic esters using O2 as the sole oxidant†
Abstract
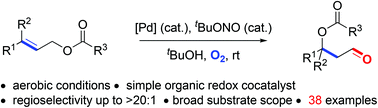
A simple and practical preparation of β-O substituted aldehydes directly from linear allylic esters is developed. Using bis(benzonitrile)palladium chloride as the catalyst and O2 as the sole oxidant, the tandem isomerization/anti-Markovnikov oxidation reaction of linear allylic esters proceeds smoothly, giving β-acyloxy aldehydes as products. This method shows a broad substrate scope and a variety of allylic alcohol derivatives bearing tertiary and even quaternary carbon centres can be selectively converted to the corresponding products. tBuOH proved to be crucial for achieving excellent regioselectivity toward the anti-Markovnikov aldehyde products.


 Please wait while we load your content...
Please wait while we load your content...