One-pot synthesis of natural-product inspired spiroindolines with anti-cancer activities†
Abstract
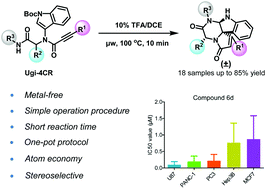
A post-Ugi/diastereoselective cascade reaction was developed to construct naturally existing spiroindolines via a facile and metal-free one-pot protocol. During the construction, a new C–C bond was formed through a selective 5-exo-dig indole cyclization on the propiolamide; and a new C–N bond was diastereoselectively formed which controlled the configuration of three chiral centers simultaneously. Screening data of several difficult-to-inhibit cancer cell lines demonstrated that (±)-6d could strongly inhibit colon cell U87 proliferation with an IC50 value of 0.19 ± 0.04 μM. Taken together, our research provided a novel and robust protocol towards the synthesis of spiroindolines and revealed their potential application as anti-cancer agents in diverse human cancer cell lines.


 Please wait while we load your content...
Please wait while we load your content...