Generalized synthesis of NaCrO2 particles for high-rate sodium ion batteries prepared by microfluidic synthesis in segmented flow†
Abstract
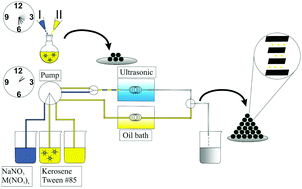
NaCrO2 particles for high-rate sodium ion batteries were prepared on a multigram scale in segmented flow from chromium nitrate and sodium nitrate using a segregated flow water-in-oil emulsion drying process. Microfluidic processing is an environmentally friendly and rapid synthetic method, which can produce large-scale industrial implementation for the production of materials with superior properties. The reaction time for NaCrO2 particles was reduced by almost one order of magnitude compared to a normal flask synthesis and by several orders of magntitude compared to a conventional solid-state reaction. In addition, it allows for an easy upscaling and was generalized for the synthesis of other layered oxides NaMO2 (M = Cr, Fe, Co, Al). The automated water-in-oil emulsion approach circumvents the diffusion limits of solid-state reactions by allowing a rapid intermixing of the components at a molecular level in submicrometer-sized micelles. A combination of Raman and nuclear magnetic resonance spectroscopy (1H, 23Na), thermal analysis, X-ray diffraction and high resolution transmission electron microscopy provided insight into the formation mechanism of NaCrO2 particles. The new synthesis method allows cathode materials of different types to be produced in a large scale, constant quality and in short reaction times in an automated manner.


 Please wait while we load your content...
Please wait while we load your content...