Hierarchical microgroove/nanopore topography regulated cell adhesion to enhance osseointegration around intraosseous implants in vivo
Abstract
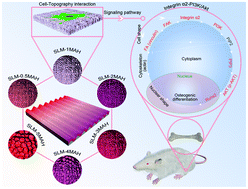
Implant surface topography plays a crucial role in achieving successful implantation. Simple and controllable surface topographical modifications are considered a promising method to accelerate bone osseointegration for biomedical applications. Moreover, comprehension of the mechanism between surface topography and cell osteogenic differentiation is vital for the manipulation of these processes to promote bone tissue regeneration. In this study, we investigated the effects of implant surfaces with various sized hierarchical microgroove/nanopore topographies on cell adhesion, osteogenesis, and their underlying mechanism both in vitro and in vivo. Our findings reveal that a titanium surface with an appropriately sized microgroove/nanopore topography (SLM-1MAH) exhibits the more satisfactory adhesive and osteogenic efficiency than the clinically used sand-blasted, large-grit, and acid-etched (SLA) surface. The underlying molecular mechanism lies in the activation of the integrin α2-PI3K-Akt signaling pathway, where the SLM-1MAH surface increased the protein expressions of integrin α2 (Itga2), phosphatidylinositol 3-kinase (PI3K), and phosphorylated serine/threonine kinase Akt (p-Akt) to enhance osteogenesis and osseointegration. Furthermore, the SLM-1MAH surface also displays better osseointegration efficiency with stronger bonding strength than that on the SLA surface. This work provides a novel strategy for implant surface topography design to improve bone–implant osseointegration.


 Please wait while we load your content...
Please wait while we load your content...