Phosphinodifluoroalkylation of alkynes using P(O)H compounds and ethyl difluoroiodoacetate†
Abstract
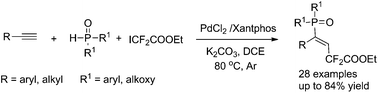
The first phosphinodifluoroalkylation of alkynes is achieved and the simultaneous addition of both difluoromethylene and phosphinoyl groups across the alkynes with high regio- and stereoselectivity is of great significance. By using palladium(II) chloride as a catalyst and Xantphos as a ligand, the reaction of P(O)H compounds, alkynes, and ethyl difluoroiodoacetate proceeds with moderate to high yields, and provides an attractive approach for the construction of (E)-γ,γ-difluoroalkenylphosphine oxides.


 Please wait while we load your content...
Please wait while we load your content...