Sortase A-mediated on-resin peptide cleavage and in situ ligation: an efficient one-pot strategy for the synthesis of functional peptides and proteins†
Abstract
Sortase A was firstly found to recognize a PEGA resin supported peptide donor containing a LPXTG motif and to ligate with a suitable acceptor containing oligo-glycine to afford conjugates in one-pot. This approach combining enzymatic on-resin cleavage, activation and in situ ligation, was employed to synthesize dual functional peptides, modify peptides with lipids, biotin and PEG, as well as protein N-terminal labeling in high efficiency.
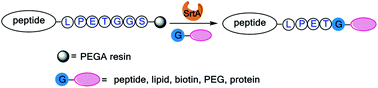


 Please wait while we load your content...
Please wait while we load your content...