A base-mediated self-propagative Lossen rearrangement of hydroxamic acids for the efficient and facile synthesis of aromatic and aliphatic primary amines†
Abstract
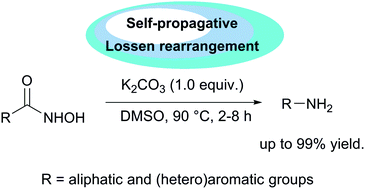
A variety of aromatic and aliphatic hydroxamic acids were converted to the corresponding primary amines via base-mediated rearrangement. This rearrangement could proceed with less than 1 equiv. of K2CO3 in polar solvents under thermal conditions with no external reagents. This rearrangement has several features including no external activating agents needed for promoting the rearrangement, less than one equivalent of a base is sufficient for the reaction, and a clean reaction in which only carbon dioxide is produced as a by-product. A self-propagating mechanism via an isocyanate intermediate is proposed and elementary reaction steps, namely, chain propagation reactions are supported by experiments.


 Please wait while we load your content...
Please wait while we load your content...