Anion directed structural diversity in zinc complexes with conformationally flexible quinazoline ligand: structural, spectral and theoretical studies†
Abstract
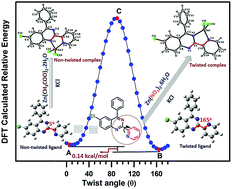
In this paper, we report the synthesis, structure and photophysical studies of four new complexes of conformationally flexible 6-chloro-4-phenyl-2-(pyridin-2-yl)quinazoline ligand (L) with Zn(II). The coordinating ability of the ligand and geometrical preferences of the resultant complexes are tuned by varying the anion of the metal salt as confirmed by structural and DFT studies. The choice of the metal salt (especially anion) directs the stabilisation of different conformations of the ligand arising out of twisting of the pyridyl ring with respect to the quinazoline ring, resulting in complexes with different nuclearity (monomer/dimer) as well as different coordination geometries (tetrahedral/trigonal bipyramidal/octahedral). Photophysical properties are also found to be tuned due to conformational changes on complexation. DFT studies on the ligand establish the conformationally stable forms as observed in the reported structures.

 Please wait while we load your content...
Please wait while we load your content...