Enhanced stability of Cu2+–ATCUN complexes under physiologically relevant conditions by insertion of structurally bulky and hydrophobic amino acid residues into the ATCUN motif†
Abstract
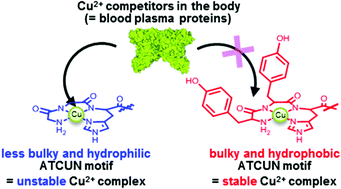
Copper complexes formed by an amino terminal Cu2+- and Ni2+-binding (ATCUN) motif have attracted attention as metallodrug candidates that cleave DNA or RNA and inactivate enzymes. Although the stability of the Cu2+–ATCUN complex under physiologically relevant conditions is a key factor for medical applications, it has remained unclear. Here we prepared a series of ATCUN peptides by inserting various amino acid residues into positions 1 and 2, and investigated the stability of the Cu2+–ATCUN complexes in aqueous solution, blood plasma, and living animals. Systematic pH titration showed that the low basicity of the N-terminal amine of the peptide stabilized the Cu2+–ATCUN complex in aqueous solution. Interestingly, the stability of 64Cu-labeled ATCUN complexes in blood plasma was significantly enhanced by the structural bulkiness and hydrophobicity of the amino acid residues at positions 1 and 2. To validate the in vivo stability, six ATCUN motifs (YYH, VVH, NNH, TTH, GGH, and DDH) were conjugated to a tumor-targeting peptide, octreotide (Oct). The stability of the 64Cu–ATCUN–Oct complexes in blood plasma showed a similar trend to that of the 64Cu–ATCUN complexes. The 64Cu–YYH–Oct complex exhibited the highest stability in blood plasma. According to the positron emission tomography and competitive blocking studies of a tumor-bearing mouse model, 64Cu–YYH–Oct specifically accumulated in tumors, suggesting that the complex was sufficiently stable to reach its target in vivo. The results show that the structural bulkiness and hydrophobicity of the residues at positions 1 and 2 are key parameters for designing metallodrugs on the basis of the Cu2+–ATCUN complex.


 Please wait while we load your content...
Please wait while we load your content...