Disulfide-bridged peptide macrobicycles from nature
Abstract
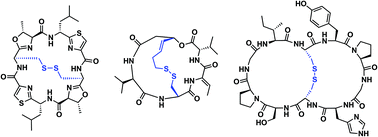
Disulfide-bridged peptide bicycles (DBPBs) are molecules that contain a transannular disulfide bridge within a macrocyclic framework. While DBPBs are precedented in nature, the development of synthetic analogues and their use as therapeutic agents has yet to realize their full potential. A series of naturally-occurring DBPBs and their respective structural and biological features is presented, followed by a description of synthetic methods used to prepare and understand these unique bicyclic systems. The synergy of high-throughput biology and synthetic chemistry should facilitate the development of novel DBPBs in the near future.

 Please wait while we load your content...
Please wait while we load your content...