Recent advances in the syntheses, transformations and applications of 1,1-dihalocyclopropanes
Abstract
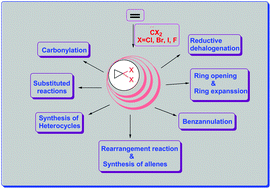
gem-Dihalocyclopropanes have wide-spread applications in organic synthesis due to their versatile chemistry. They can serve as substrates for a large range of useful materials such as natural products, alkaloids, cyclopropanes, heterocycles, aromatic ring systems etc. Normally the dihalocyclopropanes are prepared by the addition of dihalocarbene to alkene, but due to the great synthetic efficacy of gem-dihalocyclopropanes a number of methods have been developed for their synthesis. Generally gem-dihalocyclopropanes exist as strained cyclic systems with astonishing kinetic stability. They are capable of undergoing transformations leading to a variety of products which have potential applications in various synthetic organic chemistry fields.

 Please wait while we load your content...
Please wait while we load your content...