Anatase TiO2 nanocubes for fast and durable sodium ion battery anodes†
Abstract
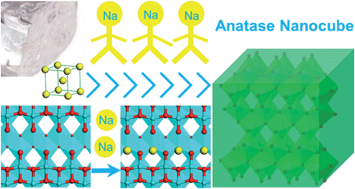
With the aim of advancing anatase TiO2 anodes for sodium ion batteries, crystalline titania nanocubes were employed and they delivered a gradually increasing capacity during the initial cycles, termed as an activation process. The number of necessary discharge–charge loops for total activation is dependent on the galvanostatic current density (about 20 cycles at 0.2 C, or 90 cycles at 1 C). A percentage of Ti3+ was detected after the activation, indicating an amount of irreversibly trapped sodium ions in the lattice. After the activation process, an excellent rate capability and outstanding cycling stability were presented. The reversible capacity reached 174, 132, and 108 mA h g−1 at rates of 1 C, 5 C, and 10 C, respectively. The capacity was sustained with a loss of less than 10% after 1000 discharge–charge cycles at a rate of 2 C or 10 C. The superior battery performance achieved by the nanocubes is related to the encircled {100} facets that are more favorable for sodium ion attachment compared to the {001} and {101} facets, as supported by first-principles calculations. From this work we can see the feasibility of optimizing electrode materials via rational surface structure construction based on theoretical calculations.

 Please wait while we load your content...
Please wait while we load your content...