Acetic acid promoted tandem cyclization of in situ generated 1,3-dipoles: stereoselective synthesis of dispiroimidazolidinyl and dispiropyrrolidinyl oxindoles with multiple chiral stereocenters†
Abstract
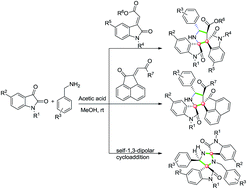
We report acetic acid promoted in situ generation of ketimine and a 1,3-dipole that results in regio- and diastereoselective formation of dispiropyrrolidineoxindoles and dispiroimidazolidineoxindoles via tandem cycloaddition. This novel one-pot reaction generates four chiral centres with two contiguous spirostereocenters in dispiropyrrolidineoxindoles, and three chiral centres with two distal spirostereocenters in dispiroimidazolidineoxindoles.

 Please wait while we load your content...
Please wait while we load your content...