Atomic layer deposited tungsten nitride thin films as a new lithium-ion battery anode†
Abstract
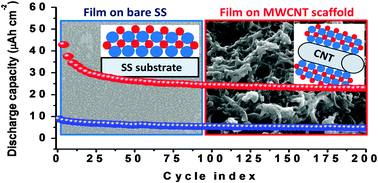
This article demonstrates the atomic layer deposition (ALD) of tungsten nitride using tungsten hexacarbonyl [W(CO)6] and ammonia [NH3] and its use as a lithium-ion battery anode. In situ quartz crystal microbalance (QCM), ellipsometry and X-ray reflectivity (XRR) measurements are carried out to confirm the self-limiting behaviour of the deposition. A saturated growth rate of ca. 0.35 Å per ALD cycle is found within a narrow temperature window of 180–195 °C. In situ Fourier transform infrared (FTIR) vibrational spectroscopy is used to determine the reaction pathways of the surface bound species after each ALD half cycle. The elemental presence and chemical composition is determined by XPS. The as-deposited material is found to be amorphous and crystallized to h-W2N upon annealing at an elevated temperature under an ammonia atmosphere. The as-deposited materials are found to be n-type, conducting with an average carrier concentration of ca. 1020 at room temperature. Electrochemical studies of the as-deposited films open up the possibility of this material to be used as an anode material in Li-ion batteries. The incorporation of MWCNTs as a scaffold layer further enhances the electrochemical storage capacity of the ALD grown tungsten nitride (WNx). Ex situ XRD analysis confirms the conversion based reaction mechanism of the as-grown material with Li under operation.

 Please wait while we load your content...
Please wait while we load your content...