A molecular dynamics study of catestatin docked on nicotinic acetylcholine receptors to identify amino acids potentially involved in the binding of chromogranin A fragments
Abstract
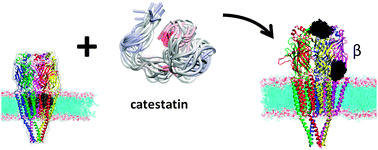
Catestatin, a cationic and hydrophobic 21-amino acid fragment of chromogranin A, is known to be a non-competitive nicotinic antagonist acting through nicotinic acetylcholine receptors (nAChRs) to inhibit catecholamine release. Since this receptor is the target of several neuronal and non-neuronal disorder prophylaxes and treatments, this study aims at the elucidation of the binding of human catestatin to the entire nAChR reconstructed in lipid bilayers by means of docking followed by full atomistic molecular dynamics simulations. The obtained results show that the minimum free energy for the binding of the peptide and the receptor attains minimal values for locations at the pore site and in the outer beta subunit. This result is consistent with previous studies showing that catestatin occludes the pore opening. A new finding is an additional even stronger binding seat at the beta subunit and that membrane presence could be an important factor. Specific amino acids involved in catestatin binding have been identified, indicating targets for point mutation studies. In addition to improving the understanding of the interaction between the peptide and muscle-type and even other nAChR subtypes, the results of this study provide directions for future peptidomimetic research.

 Please wait while we load your content...
Please wait while we load your content...