Copper-catalyzed redox-neutral C–H amination with amidoximes†
Abstract
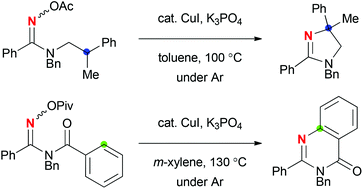
CuI-catalyzed reactions of N-alkylamidoximes afforded dihydroimidazoles via sp3 C–H amination. On the other hand, the reactions of N-benzoylamidoximes resulted in sp2 C–H amination to form quinazolinones. The reaction mechanisms could be characterized as a redox-neutral radical pathway including a Cu(I)–Cu(II) redox catalytic cycle.

 Please wait while we load your content...
Please wait while we load your content...