Carbon–fluorine bond cleavage in fluoroarenes via a niobium(iii) imido complex: from stoichiometric to catalytic hydrodefluorination†
Abstract
The mechanism of activation of C–F bonds in fluoroarenes using a well-defined niobium(III) imido complex has been investigated. The Nb(III) arene species [BDI]Nb(NtBu)(η6-C6H6), 1, (BDI = β-diketimate), reacts stoichiometrically with fluoroarenes to yield niobium(V) aryl fluorides. Spectroscopic analysis supported by DFT calculations revealed the critical involvement of a Nb(III) fluoroarene-bound species. In contrast to previous reports of related reactivity, we found that perfluorinated arenes (i.e. those normally assumed to bear more ‘activated’ C–F bonds) are, in the present system, much less reactive towards C–F bond cleavage than mono- or difluoro-substituted arenes. In addition to demonstrating stoichiometric hydrodefluorination reactions, we also describe an efficient and mild hydrodefluorination of mono- and di-fluoroarenes that is catalytic in niobium.
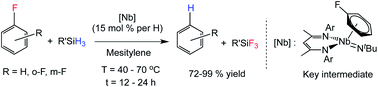

 Please wait while we load your content...
Please wait while we load your content...