A simple and effective method for controllable synthesis of silver and silver oxide nanocrystals†
Abstract
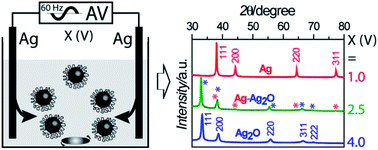
Silver (Ag) and silver oxide (Ag2O) colloidal nanocrystals are easily interconvertable and important for many similar applications; however, they have not been synthesized using the same method. Here we report a simple and effective method for synthesizing well-dispersed, hydrophilic, and colloidal Ag, Ag2O, and Ag–Ag2O mixed nanocrystals, which are 10–50 nm in diameter, sphere-like, and passivated with the same stabilizer polyvinylpyrrolidone (PVP). The method is denoted as alternating voltage induced electrochemical synthesis (AVIES). It involves only a transformer, two pieces of identical Ag wires, a stirring plate, and a PVP-containing electrolyte solution. The control of Ag and/or Ag2O nanocrystals is achieved by tuning the electrolyte identity, the stirring rate, and the applied voltage. Toward exploring practical applications of heterogeneous catalysis and antibacterial therapy, we have employed the synthesized NCs to catalyze the reduction of 4-nitrophenol and fabricated Ag NCs-loaded electrospun carbon fibers, respectively. Moreover, the revealed crystal-formation mechanism is surprising and interesting: the intermediate states for producing Ag nanocrystals are Ag2O nano-islands formed in situ on the electrode, and not the Ag+ ions in solution formed in situ through electrochemical etching.

 Please wait while we load your content...
Please wait while we load your content...