Exchange of cysteamine, thiol ligand in binuclear cationic tetranitrosyl iron complex, for glutathione†
Abstract
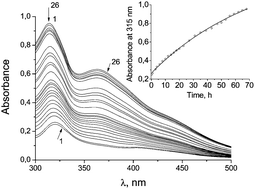
This paper describes the comparative study of the decomposition of two iron nitrosyl complexes (NICs) with a cysteamine thiolate ligand {Fe2[S(CH2)2NH3]2(NO)4}SO4·2.5H2O (I) and a glutathione (GSH)-ligand, [Fe2(SC10H17N3O6)2(NO)4]SO4·2H2O (II), which spontaneously evolve NO in aqueous medium. NO formation was measured by using a spectrophotometric method by the formation of a hemoglobin (Hb)–NO complex. Spectrophotometry and mass-spectrometry methods have firmly shown that the cysteamine ligands are exchanged for 2 GS− during decomposition of 1.5 × 10−4 M (I) in the presence of 10−3 M GSH, with 77% yield at 68 h. As has been established, such behaviour is caused by the resistance of (II) to decomposition due to the higher affinity of iron towards GSH in the complex. The discovered reaction may impede S-glutathionation of the essential enzyme systems the presence of (I) and is important for metabolism of NICs, connected with their anti-tumor activity.

 Please wait while we load your content...
Please wait while we load your content...