A tumor-targeting dextran–apoprotein conjugate integrated with enediyne chromophore shows highly potent antitumor efficacy†
Abstract
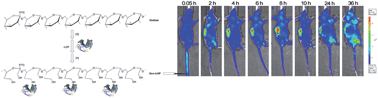
A novel polymer–protein conjugate, Dex–rLDP, was prepared through conjugation of recombinant apoprotein (rLDP) of antitumor antibiotic lidamycin (LDM) to a macromolecular carrier, dextran T40, by the periodate oxidation–hydroboron reduction method. Its structure was verified by means of SDS-PAGE, HPLC, FT-IR, CD and MALDI-TOF MS. The weight-average molecular weight (Mw) of the glycoconjugate was 66.6 kDa, determined by MALDI-TOF MS, suggesting that approximately 3 mol of rLDP was attached per mole of oxidized dextran. The particle size, zeta potential and thermal stability of the dextranated conjugate were further characterized by TEM, DLS, and DSC. The DSC analysis revealed that dextranation can markedly enhance the thermal stability of the recombinant protein. To empower the conjugate with highly potent cytotoxicity, the resulting Dex–rLDP was then assembled with the active enediyne (AE) chromophore of LDM to generate an enediyne-energized conjugate, namely Dex–rLDP–AE. In vitro MTT assay clearly indicated that the cytotoxicity of Dex–rLDP–AE was at least an order of magnitude higher than that of free LDM. At tolerable doses, Dex–rLDP–AE markedly suppressed the growth of human carcinoma xenografts and transplantable murine hepatoma. Notably, selective accumulation and retention of the fluorescently labeled Dex–rLDP within the tumor was detected by in vivo fluorescence imaging in tumor-bearing mice. The observed results indicate that this cross-linking strategy offers important implications for controlled and targeted drug delivery to solid tumors, and the enediyne-energized dextran–apoprotein conjugate is a potentially promising candidate for tumor targeted therapy.

 Please wait while we load your content...
Please wait while we load your content...