Bioelectrocatalytic reduction of O2 at a supramolecularly associated laccase electrode†
Abstract
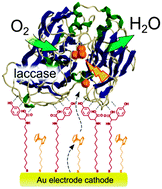
In order to improve the bioelectrocatalytic reduction of dioxygen, a method for the supramolecular immobilization of Trametes versicolor laccase is reported. The immobilization support consisted of a self-assembled monolayer (SAM) of mercaptoundecanoic acid tyrosine amide on gold electrodes. To mediate the electron transfer between the enzyme and the electrode surface, ferrocenehexanethiol was introduced into the SAM as a second component. The resulting system was studied by cyclic voltammetry. Reduction of dioxygen started at 0.80 V, with a reduction peak at 0.52 V and a decrease in its intensity was observed when the solution was deaerated. The achieved current density for dioxygen reduction (23 μA cm−2, open to air) suggests a high surface density of active enzyme prone to bioelectrocatalytic activity. This system offers new insights into the supramolecular design of biofuel cells.

 Please wait while we load your content...
Please wait while we load your content...