Metal(ii) complexes based on 4-(2,6-di(pyridin-4-yl)pyridin-4-yl)benzonitrile: structures and electrocatalysis in hydrogen evolution reaction from water†
Abstract
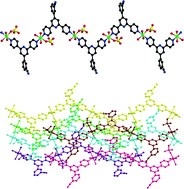
Using a novel rigid ditopic ligand, 4-(2,6-di(pyridin-4-yl)pyridin-4-yl)benzonitrile (L), three metal(II) complexes formulated as M2L2(SO4)2(H2O)6·H2O (M = Ni (1), Co (2) and Cd (3)) have been solvothermally synthesized and structurally characterized by single-crystal X-ray diffraction. The three complexes are isostructural except for the different metal(II) ions in the structures. They all show a one-dimensional (1D) chain, in which the L ligand acts as a μ2-bridge linking two metal(II) centers. Different chains are connected by strong H bonds and π–π stacking interactions into a three-dimensional (3D) supramolecular architecture. Among the three complexes, the Ni complex 1 and the Co complex 2 can act as the electrocatalysts for the hydrogen evolution reaction (HER) from water, and the Co complex 2 shows better electrocatalytic activity. In the present work, the graphene cannot catalyze the HER; however, the complex 1/graphene composite shows similar electrocatalytic activity to complex 1. The three complexes show different UV-vis absorption values, photoluminescence properties and thermal stabilities.

 Please wait while we load your content...
Please wait while we load your content...