Synthesis of single crystalline sub-micron rutile TiO2 rods using hydrothermal treatment in acidic media†
Abstract
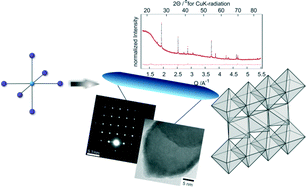
Size engineered rutile sub-micron rods were obtained from nanostructured titania under acidic conditions. The synthesis was performed by hydrothermal treatment starting from TiO2-P25 and HCl. The synthesis proceeds in less than two hours and can be up-scaled to several grams in a one-pot reaction by increasing the reaction time. The product is single-phase, and the particles are single crystalline as confirmed by electron diffraction and powder X-ray diffraction analysis. The length of the particles can be varied over a wide range from 100 nm to 1.3 μm by changing the acid concentration. Particle growth is proposed to proceed by a dissolution-recrystallization process via soluble [TiCl6]2− or partially hydrated/hydroxylated species.

 Please wait while we load your content...
Please wait while we load your content...