TICT fluorescence of N-borylated 2,5-diarylpyrroles: a gear like dual motion in the excited state†
Abstract
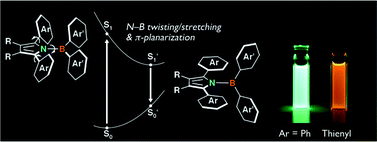
A significantly red-shifted fluorescence and a high fluorescence quantum yield for the emission from the twisted intramolecular charge transfer (TICT) state are attained in new aminoboranes, N-borylated 2,5-diarylpyrroles. A gear like dual structural motion in the excited state is responsible for their large Stokes shifts.
- This article is part of the themed collection: Boranes and borohydrides

 Please wait while we load your content...
Please wait while we load your content...