Late stage trifluoromethylthiolation strategies for organic compounds
Abstract
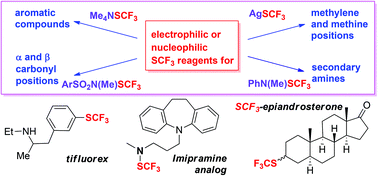
Substitution by the CF3S group allows for an increase in lipophilicity and electron-withdrawing properties along with an improvement in the bioavailability of medicinal targets; consequently, the late stage introduction of CF3S moieties into medicinal scaffolds is a sought-after strategy in synthetic organic chemistry. Different newly-developed electrophilic and nucleophilic reagents are used to effect the trifluoromethylthiolation of (hetero)aromatic compounds, aliphatic compounds (alkyl, alkenyl, alkynyl substrates), the trifluoromethylthiolation at the α- and β-carbonyl positions, and heteroatoms (N- and S-). Such reactions can involve homolytic substitutions, or functional-group substitutions (ipso). Addition reactions of electrophilic reagents to double and triple bonds followed by ring-cyclizations will be shown to yield relevant CF3S-substituted heteroaromatic compounds with relevant pharmacological action.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...