Enantioselective synthesis of fluorinated branched allylic compounds via Ir-catalyzed allylations of functionalized fluorinated methylene derivatives†
Abstract
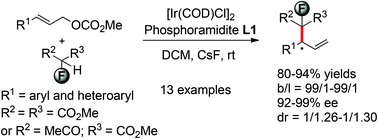
Enantioselective introduction of the functionalized monofluorinated methylenes into the allylic fragment under Ir catalysis has been realized, which gave the fluorinated branched allyl products in good to high yields with excellent regio- and enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...