Photoredox synthesis of 6- and 7-membered ring scaffolds via N-centered radicals†
Abstract
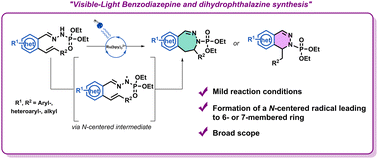
N-Containing heterocycles are important scaffolds due to their ubiquitous presence in bioactive compounds. Their synthesis has been considered as an important research field. In this work we report the access to 6- and 7-membered rings via a photoinduced strategy. To our knowledge, this work represents the first exemple of photo-induced 7-endo-trig cyclization with N-centered radicals.
- This article is part of the themed collection: Celebrating 10 years of ChemComm Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...