Nonaqueous electrolyte with dual-cations for high-voltage and long-life zinc batteries†
Abstract
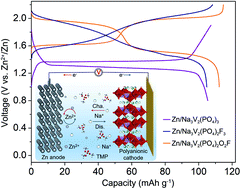
Rechargeable zinc-based batteries (RZBs) are attracting extensive attention because of their considerable energy density, high safety and low cost, but generally suffer from the uncontrollable dendrite formation and low reversibility of zinc (Zn) anodes along with the limited voltage and unsatisfactory cyclability of current oxide cathodes in aqueous electrolytes. Here, we report a nonaqueous phosphate-based electrolyte with dual cations consisting of 0.5 M Zn2+ and 1.0 M Na+ in trimethyl phosphate (TMP) solvent. The formulated electrolyte features a fire-extinguishing ability, an expanded anodic stability up to 2.8 V versus Zn, and a good compatibility to both Zn anode and polyanionic cathodes (e.g., Na3V2(PO4)2O2F (NVPOF), Na3V2(PO4)2F3 (NVPF), and Na3V2(PO4)3 (NVP)). A dendrite-free and high-coulombic-efficiency cycling of Zn anode with a high cycling stability (over 5000 h) is characterized. As a proof-of-concept, a new configuration of RZB by employing a high-voltage NVPOF cathode, a Zn anode, and the dual-cation electrolyte shows an average output voltage of 1.8 V with an energy density of 203 W h kg−1 (based on the cathode) and a long-term cyclability with 83.5% retention over 1000 cycles. This battery chemistry operates through the reversible extraction/insertion of Na+ from/into the NVPOF cathode and electrochemical deposition/dissolution of Zn at the anode. Impressively, significantly improved performances of other polyanionic cathodes (e.g., NVPF and NVP) have also been realized in the formulated electrolyte. The present work broadens the horizon of electrolytes and cathode materials for Zn battery chemistries.


 Please wait while we load your content...
Please wait while we load your content...