A robust self-stabilized electrode based on Al-based metallic glasses for a highly efficient hydrogen evolution reaction†
Abstract
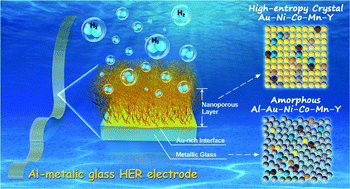
An electrode with low performance or limited endurance for water splitting hydrogen generation has been the key limitation for application of hydrogen in contemporary clean-energy technologies. In this paper, an Al80Ni6Co3Mn3Y5Au3 metallic glass ribbon with outstanding catalytic activity in the hydrogen evolution reaction (HER) in acidic solutions is fabricated for the first time. Its overpotential is about 70 mV @ 10 mA cm−2 and Tafel slope is about 39 mV dec−1, which are comparable to those of commercial noble Pt/C electrodes (33 mV @ 10 mA cm−2 and 38 mV dec−1). Such a high catalytic reactivity is attributed to the synergic effect of multiple elements that disperse atomically homogeneously on the nanoporous surface. The outstanding catalytic activity persists and even becomes much better over a long-time reaction, which is attributed to the formation of a protective Au-rich layer in the interface. The Al-based metallic glass ribbon is flexible with high yield strength, large elasticity and good electrical conductivity, which are desirable and promising characteristics for a free-standing catalytic electrode in the HER.


 Please wait while we load your content...
Please wait while we load your content...