Designing solution chemistries for the low-temperature synthesis of sulfide-based solid electrolytes†
Abstract
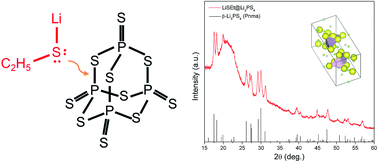
Developing synthesis methods for high quality solid electrolytes has been a key issue for enabling all-solid-state batteries. As compared to conventional methods using mechanical ball milling, liquid-phase synthesis methods would provide a facile way to produce solid electrolytes by reducing the reaction time and heating temperature. The simplified process is also potentially applicable to scalable manufacturing. Here, we introduce a new solution-based synthesis method for an Li2S–P2S5 solid electrolyte by adding a nucleophilic agent, LiSC2H5. The strong nucleophile can break the P–S bonds of P2S5, fully dissolving the P2S5 in tetrahydrofuran (THF) and forming soluble intermediates. The modified synthesis protocol provides kinetically favorable conditions for P2S5 to react with the insoluble Li2S, demonstrating the formation of a high quality β-Li3PS4 solid electrolyte (1.32 × 10−4 S cm−1) with a uniform particle shape.


 Please wait while we load your content...
Please wait while we load your content...
