A phenol-fused tetrathiafulvalene: modulation of hydrogen-bond patterns and electrical conductivity in the charge-transfer salt†
Abstract
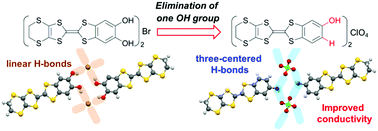
A tetrathiafulvalene (TTF) derivative fused with a phenol moiety, PhOH-EDT-TTF, was designed and synthesized as a new electron-donor molecule with hydrogen-bonding (H-bonding) abilities. Electrochemical oxidation of this donor molecule in the presence of Bu4NClO4 gave a charge-transfer salt, β-(PhOH-EDT-TTF)2ClO4, that contains PhOH-EDT-TTF+0.5 and ClO4− in a 2 : 1 ratio. In this salt, the donor and anion molecules are connected with three-centered H-bonds through the phenol hydroxy group, which is in contrast to the linear H-bonds observed in an analogue salt based on the catechol-fused TTF. This variation in the donor-anion H-bonds modulated the arrangement of the donor molecules in the conducting layer, leading to better conducting properties than those of the analogue salt.
- This article is part of the themed collection: Pi conjugated system bricolage (figuration) toward functional organic molecular systems


 Please wait while we load your content...
Please wait while we load your content...