Modulation of luminescence chromic behaviors and environment-responsive intensity changes by substituents in bis-o-carborane-substituted conjugated molecules†
Abstract
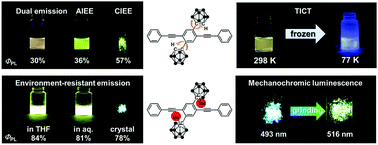
Two types of multi-functional emissive bis-o-carborane-substituted 1,4-bis(phenylethynyl)benzene molecules were synthesized, and their optical properties were investigated in detail. The pristine o-carborane-substituted molecule CBH simultaneously exhibited dual emission from the locally excited (LE) and twisted intramolecular charge transfer (TICT) states in solution. Originating from changes in the intensity ratios between both emission bands, clear solvatochromic and thermochromic behaviors were observed. Surprisingly, TICT emission was observed even in the solid state. Aggregation- and crystallization-induced emission enhancement (AIEE and CIEE, respectively) were also presented by CBH. These solid-state emission enhancements could be derived from the suppression of aggregation-caused quenching (ACQ) by the bulky cage structure and the spherical shape of o-carborane. Next, we also synthesized the methyl-substituted derivative (CBMe) and found environment-resistant highly-efficient emission in both the solution and solid states. Finally, CBMe presented mechanochromic luminescence in the solid state. The substituent effects on the optical properties are discussed.
- This article is part of the themed collection: Recent Progress on Aggregation-Induced Emission


 Please wait while we load your content...
Please wait while we load your content...