Preparation of pyrrolizinone derivatives via sequential transformations of cyclic allyl imides: synthesis of quinolactacide and marinamide†
Abstract
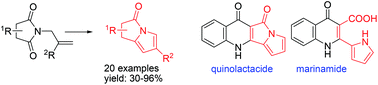
A facile synthetic route has been developed for the preparation of pyrrolizinone derivatives employing N-allyl imides as starting materials. The nucleophilic addition of a vinyl Grignard reagent/RCM/elimination sequence afforded pyrrolizinones in good yields and has been applied for the preparation of naturally occurring quinolactacide and marinamide.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...