Synthesis of medium-sized aryl-fused nitrogenous heterocycles via sequential aryne aza-Claisen rearrangement/ring-closing metathesis†
Abstract
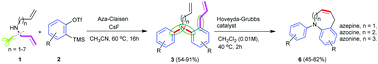
The reaction of arynes and secondary allylamines furnished ortho-allyl-substituted N-arylanilines via an aza-Claisen rearrangement. In this transformation, the sequential formation of C–C and C–N bonds occurred by involving two aryne molecules under metal-free reaction conditions to provide moderate to good yields of the products. The obtained ortho-allyl-substituted N-arylaniline derivatives were further converted into aryl-fused medium-sized (7–9) nitrogenous heterocyclic molecules such as azepines, azocines and azonines via ring-closing metathesis (RCM).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...